
The Moles of Chalk Lab is a common experiment in chemistry classrooms used to introduce students to the concept of moles and stoichiometry. In this lab, students determine the number of moles of calcium carbonate (chalk) in a given sample by reacting it with an excess of hydrochloric acid.
In the lab, students first measure and record the mass of the chalk sample. They then add the chalk to a flask and add an excess of hydrochloric acid. The reaction between the chalk and the acid produces carbon dioxide gas, which is collected in a gas syringe or a gas collection system. By measuring the volume of gas produced and knowing the molar volume of an ideal gas at standard temperature and pressure, students can determine the number of moles of chalk in the sample.
The answer key for this lab provides students with the expected results and calculations. It allows students to compare their own data and calculations with the expected values, helping them to identify any errors or deviations from the expected results. The answer key also provides explanations and step-by-step calculations, reinforcing the understanding of the mole concept and stoichiometry.
The Moles of Chalk Lab is an important hands-on activity that helps students develop critical thinking and problem-solving skills. By analyzing the data and comparing it to the answer key, students can learn from their mistakes and improve their understanding of mole calculations. This lab also emphasizes the importance of accurate measurements and proper experimental techniques in chemistry.
Moles of Chalk Lab Answer Key
In the “Moles of Chalk Lab”, students were tasked with determining the number of moles of calcium carbonate in a sample of chalk. They performed a simple acid-base titration using hydrochloric acid and phenolphthalein as an indicator.
The first step in the lab was to measure out a sample of chalk and determine its mass. This was done using a balance, and the mass of the chalk was recorded. Next, the students crushed the chalk into a fine powder and added it to a flask. They then added a known volume of hydrochloric acid to the flask, causing a reaction to occur. The acid reacted with the calcium carbonate in the chalk, producing carbon dioxide gas.
The carbon dioxide gas was collected in a gas syringe and its volume was measured. From this volume, the students were able to calculate the number of moles of carbon dioxide gas produced. Since the reaction between calcium carbonate and hydrochloric acid is 1:1, the number of moles of carbon dioxide gas is equal to the number of moles of calcium carbonate in the original sample of chalk.
The students then used the balanced chemical equation for the reaction between calcium carbonate and hydrochloric acid to calculate the number of moles of calcium carbonate. They also used the molar mass of calcium carbonate to convert moles into grams. This allowed them to determine the mass of calcium carbonate in the sample of chalk.
Overall, the “Moles of Chalk Lab” provided an opportunity for students to practice their skills in measuring mass, performing a titration, and calculating moles and molar mass. It also helped them understand the concept of stoichiometry and the relationship between reactants and products in a chemical reaction.
The Purpose of the Chalk Lab Experiment

The chalk lab experiment was conducted to determine the number of moles of chalk present in a given sample. This information is crucial for various scientific and industrial applications, as it helps in calculating the amount of reactants and products involved in a chemical reaction. Additionally, determining the moles of chalk allows scientists to understand the composition, structure, and properties of the substance, which further contributes to advancements in fields such as material science and geology.
During the experiment, students measured the mass of the chalk sample using a digital balance. The molar mass of chalk, which is composed primarily of calcium carbonate (CaCO3), was calculated beforehand. By dividing the mass of the sample by its molar mass, students could obtain the number of moles present in the chalk. This value served as a quantitative measure of the amount of chalk, facilitating further analyses and comparisons.
The results obtained from the chalk lab experiment provided valuable information that could be used in various ways. For instance, researchers studying chemical reactions could utilize the data to determine the stoichiometry of a reaction involving chalk, helping them accurately predict the amounts of other substances needed for the reaction. Industrial applications could benefit from this data by ensuring precise measurements and formulations in processes involving chalk, such as the manufacturing of cement or the use of chalk as a coloring agent in paints and dyes.
Overall, the purpose of the chalk lab experiment was to quantitatively determine the number of moles of chalk in a given sample. This information has broad implications in scientific research, industrial processes, and material development, showcasing the importance of such experiments in various fields.
Experimental Setup and Materials
The experimental setup for the Moles of Chalk lab requires the following materials:
- Beakers (250 mL)
- Burette
- Pipette
- Burette clamp
- Weighing balance
- Chalk samples
- Hydrochloric acid solution
- Distilled water
- Indicator solution (phenolphthalein)
- Filter paper
The beakers are used to hold the solutions during the experiment. The burette is used for measuring and dispensing the hydrochloric acid solution. The pipette is used for measuring and transferring small volumes of the indicator solution. The burette clamp is used to securely hold the burette in place during titration.
The weighing balance is used to measure the mass of the chalk samples. The chalk samples are the substances being analyzed in this experiment. The hydrochloric acid solution is the reactant used to react with the chalk samples. The distilled water is used to dilute the hydrochloric acid solution as needed. The indicator solution, phenolphthalein, is used to visually indicate when the reaction between the chalk and acid is complete. The filter paper is used to filter any solid residue left after the reaction.
Overall, the experimental setup and materials in the Moles of Chalk lab are carefully chosen to ensure accurate measurements and reliable results. Each component has a specific role and contributes to the successful completion of the experiment.
Step-by-Step Procedure for the Chalk Lab Experiment
The chalk lab experiment aims to determine the number of moles in a sample of chalk. This step-by-step procedure will guide you through the experiment and help you obtain accurate results.
Materials:
- Sample of chalk
- Balance
- Beaker
- Hydrochloric acid
- Graduated cylinder
- Burette
- Phenolphthalein indicator
- Spatula
- Pipette
Procedure:
- Weigh the empty beaker using the balance and record the measurement.
- Add a known mass of chalk to the beaker and reweigh it. Record the mass of the chalk.
- Measure a known volume of hydrochloric acid using the graduated cylinder.
- Add the hydrochloric acid to the beaker containing the chalk.
- Wait for the reaction to take place. The chalk should dissolve in the acid.
- Once the reaction is complete, add a few drops of phenolphthalein indicator to the beaker.
- Fill the burette with a solution of sodium hydroxide.
- Add the sodium hydroxide solution drop by drop to the beaker while stirring continuously.
- Observe the color change in the beaker. The color will change from clear to pink.
- Continue adding the sodium hydroxide solution until the pink color disappears and the solution turns clear again. Record the volume of sodium hydroxide solution used.
By following this step-by-step procedure, you will be able to determine the number of moles in the sample of chalk. This experiment helps to understand the concept of moles and demonstrates the reaction between calcium carbonate in chalk and hydrochloric acid. It also introduces the concept of titration using sodium hydroxide solution.
Data Collection and Analysis
During the Moles of Chalk Lab, various measurements and observations were collected to determine the number of moles of calcium carbonate (CaCO3) in a piece of chalk. These data points were crucial for accurately calculating the molar mass of the compound and determining its empirical formula.
Mass Measurements:
- The mass of the empty crucible was measured using an electronic balance, and it served as the initial baseline for mass measurements.
- A small piece of chalk was added to the crucible, and the mass of the crucible with the chalk was recorded.
- The crucible with the chalk was heated over a Bunsen burner to drive off any volatile substances, and the mass of the crucible with the remaining chalk residue was measured.
Other Measurements and Observations:
- A small amount of hydrochloric acid (HCl) was added to the crucible to react with the chalk residue. The reaction produced carbon dioxide gas (CO2), which was observed as effervescence.
- To ensure complete reaction, the crucible was heated and the remaining chalk residue was weighed again.
- The volume of carbon dioxide gas produced during the reaction was measured using a gas syringe.
Once all the necessary measurements and observations were recorded, the collected data was then analyzed to calculate the number of moles of CaCO3 in the original piece of chalk. The moles of chalk were determined by using the molar mass of CaCO3 and the mass of the chalk residue. The empirical formula of the chalk sample was then determined based on the moles of calcium ions (Ca2+) and carbonate ions (CO32-) present in the compound.
The data collection and analysis process allowed for the quantitative determination of the composition of the chalk sample. The results obtained from this lab provided valuable insights into the molecular structure and composition of calcium carbonate, as well as the stoichiometry of the reaction between calcium carbonate and hydrochloric acid. This data is crucial for further understanding the chemical properties and behavior of chalk in various applications.
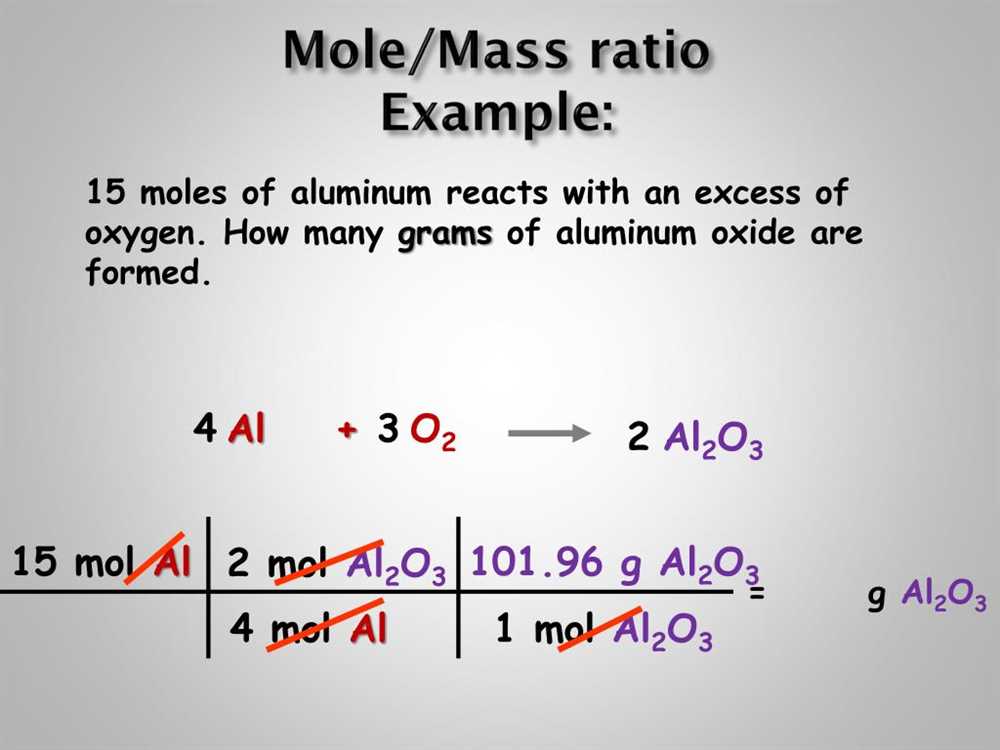
Calculating the Number of Moles of Chalk
When conducting a lab that involves measuring the mass of a chalk sample and determining the number of moles, it is important to follow a specific calculation process. This process involves converting the mass of the chalk sample into moles by using the molar mass of calcium carbonate, which is the chemical compound found in chalk.
The first step in calculating the number of moles of chalk is to measure the mass of the chalk sample using a balance. Make sure to record the mass in grams, as this is the standard unit for measuring mass in chemistry. Once you have the mass of the chalk sample, you can proceed to the next step.
Next, you need to determine the molar mass of calcium carbonate. The molar mass is the mass of one mole of a substance and is usually expressed in grams per mole (g/mol). The molar mass of calcium carbonate can be calculated by adding up the atomic masses of each element in the compound. In the case of calcium carbonate, it consists of one calcium atom (Ca) with a molar mass of 40.08 g/mol, one carbon atom (C) with a molar mass of 12.01 g/mol, and three oxygen atoms (O) with a molar mass of 16.00 g/mol each. Therefore, the molar mass of calcium carbonate is 40.08 g/mol + 12.01 g/mol + (3 * 16.00 g/mol) = 100.09 g/mol.
Finally, you can calculate the number of moles of chalk by dividing the mass of the chalk sample by the molar mass of calcium carbonate. This can be done using the formula:
Number of moles = Mass of chalk sample (g) / Molar mass of calcium carbonate (g/mol)
By substituting the values you obtained for the mass of the chalk sample and the molar mass of calcium carbonate, you can easily calculate the number of moles of chalk. This calculation is important in chemistry as it allows for precise measurements and comparisons of substances.
Interpretation of Results and Discussion
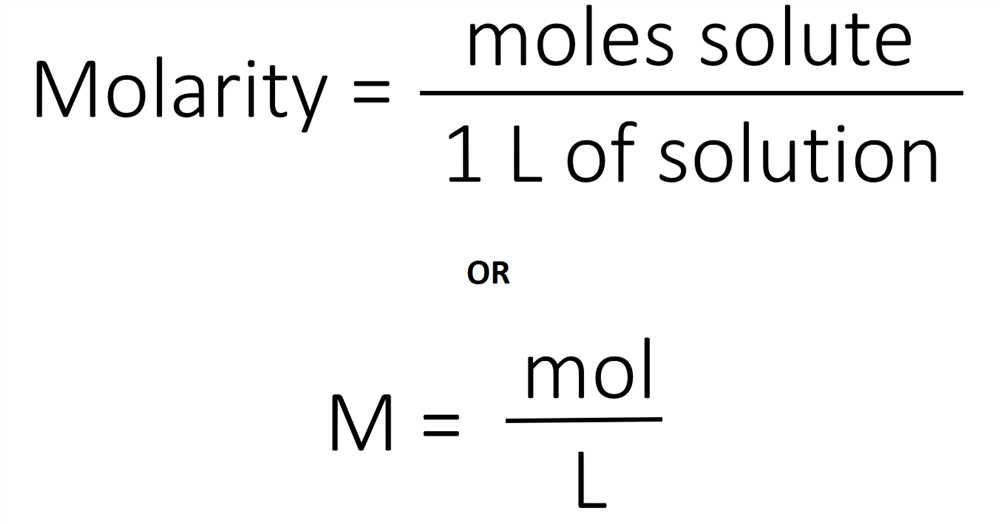
After completing the “Moles of Chalk” lab, the data obtained can now be analyzed to draw conclusions. The experiment aimed to determine the number of moles of carbon dioxide produced when chalk reacts with hydrochloric acid. By measuring the mass of chalk before and after the reaction, as well as the volume of carbon dioxide gas produced, it is possible to calculate the moles of carbon dioxide produced.
The mass loss of chalk: The mass of chalk before the reaction was recorded, and after the reaction, the mass was measured again. The difference in mass indicates how much chalk was consumed during the reaction. From this, we can calculate the moles of chalk that reacted, using the molar mass of chalk as a conversion factor.
The volume of carbon dioxide: The volume of carbon dioxide gas produced during the reaction was collected in a gas syringe. This volume can be directly used to calculate the moles of carbon dioxide produced, using the ideal gas law.
With the moles of chalk reacted and the moles of carbon dioxide produced, it is possible to determine the stoichiometry of the reaction. From the balanced chemical equation for the reaction between chalk and hydrochloric acid, the ratio of moles of chalk to moles of carbon dioxide can be determined. This ratio can be compared to the experimental results to evaluate the accuracy of the data and to identify any sources of error.
The discussion of the results should include an analysis of the experimental procedure, including any potential sources of error. It is important to consider factors such as incomplete reactions, impurities in the chalk, or errors in measurement. Additionally, any limitations of the experimental setup or equipment used should be addressed.
In conclusion, the interpretation of the results and discussion of the “Moles of Chalk” lab should provide a thorough analysis of the data obtained. By calculating the moles of chalk and carbon dioxide, as well as considering the stoichiometry of the reaction, it is possible to evaluate the accuracy of the experimental results and identify any areas for improvement in future experiments.