The Mystery Gang, a famous group of detectives, has once again stunned the world with their ability to solve seemingly unsolvable mysteries. One of their recent cases involved deciphering the empirical formulas of various substances, revealing the secrets hidden within. The gang’s expertise in chemistry and deduction allowed them to unlock the mysteries behind these formulas, shedding light on their composition and properties.
Empirical formulas are the simplest form of chemical formulas, showing the ratio of elements present in a compound. They provide valuable insights into the elemental makeup of substances without revealing their molecular structure. The Mystery Gang’s skillful analysis of these formulas helped them unravel the enigmatic nature of the compounds, paving the way for groundbreaking discoveries.
By examining empirical formulas, the Mystery Gang was able to deduce the elemental composition of substances, identifying the key ingredients responsible for their unique properties. Whether it was a mysterious potion, an unknown powder, or a hidden substance, the gang’s attention to detail and scientific knowledge enabled them to connect the dots and solve the puzzles presented to them.
Through their extraordinary abilities, the Mystery Gang demonstrated the power of empirical formulas in unraveling the mysteries of chemistry. Their dedication to solving perplexing cases showcased not only their investigative skills but also their scientific expertise. By deciphering and understanding these formulas, the gang opened new doors to a world of discovery and learning, leaving us in awe of their remarkable achievements.
Mystery Gang Empirical Formula Answers
When it comes to solving mysteries, the Mystery Gang is always on the case. Using their intelligence, deductive skills, and teamwork, they manage to unravel secrets and bring justice to the world. One of the most intriguing aspects of their adventures is figuring out the empirical formulas of mysterious substances or compounds they come across.
The empirical formula provides the simplest ratio of atoms present in a compound. It is obtained through analysis and experimentation. The Mystery Gang often encounters unknown substances or materials that are pivotal to solving the mystery at hand. By determining their empirical formulas, they can gain crucial insights into the nature and behavior of these substances.
Some examples of empirical formula answers:
- Mystery Solution X: Through careful analysis, the Mystery Gang discovers that the empirical formula of Solution X is CH2O. This suggests that the solution contains carbon, hydrogen, and oxygen in a 1:2:1 ratio. Further investigation reveals that Solution X is a sugar compound.
- Mysterious Metal Alloy Y: After conducting experiments, the Mystery Gang finds that the empirical formula of Alloy Y is Fe3O4. This indicates that the alloy consists of iron (Fe) and oxygen (O) atoms in a 3:4 ratio. It turns out that Alloy Y is a type of magnetite, a naturally occurring magnetic mineral.
- Enigmatic Substance Z: In their pursuit of unraveling a cryptic case, the Mystery Gang encounters Substance Z. By analyzing its properties, they determine its empirical formula to be N2O5. This implies that Substance Z contains two nitrogen (N) atoms and five oxygen (O) atoms. Eventually, they discover that Substance Z is a highly reactive and volatile compound often used in chemical reactions.
The empirical formula answers provide valuable insights into the composition of the mysterious substances encountered by the Mystery Gang. Armed with this knowledge, they can make informed decisions and connect the dots to solve the mysteries they encounter.
What is an Empirical Formula?
The empirical formula is the simplest ratio of the different elements present in a chemical compound. It represents the relative number of atoms of each element in the compound. The empirical formula does not provide the exact number of atoms, but rather the smallest whole number ratio in which the elements are present in the compound. It is a fundamental concept used in chemistry to describe the composition of compounds.
To determine the empirical formula of a compound, the percentage composition data is required. The percentage composition refers to the mass percent of each element in the compound. By converting the mass percent to moles and finding the lowest whole number ratio between the elements, the empirical formula can be determined.
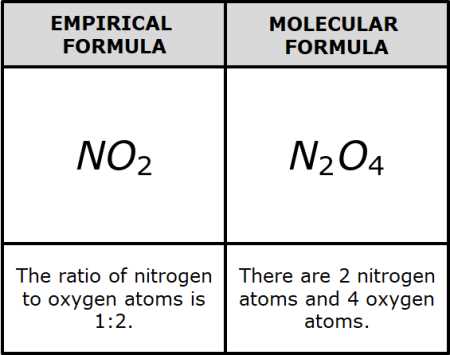
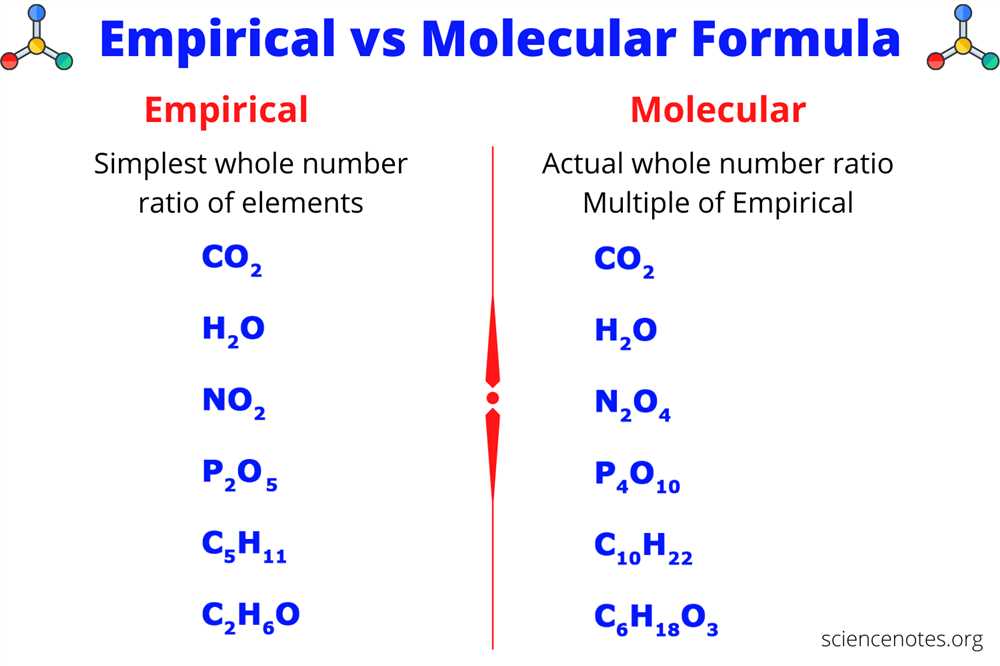
In some cases, the empirical formula may be the same as the molecular formula. For example, the empirical formula and molecular formula of hydrogen peroxide are both H2O2. However, in many cases, the empirical formula is a simplified version of the molecular formula. For instance, the molecular formula of glucose is C6H12O6, but its empirical formula is CH2O.
The empirical formula is an essential tool in chemistry as it helps determine the basic composition of compounds. It provides valuable information about the elements present in a compound and their relative proportions. By knowing the empirical formula, scientists can further study the properties and behavior of compounds in various chemical reactions.
The Role of Empirical Formulas in Chemistry
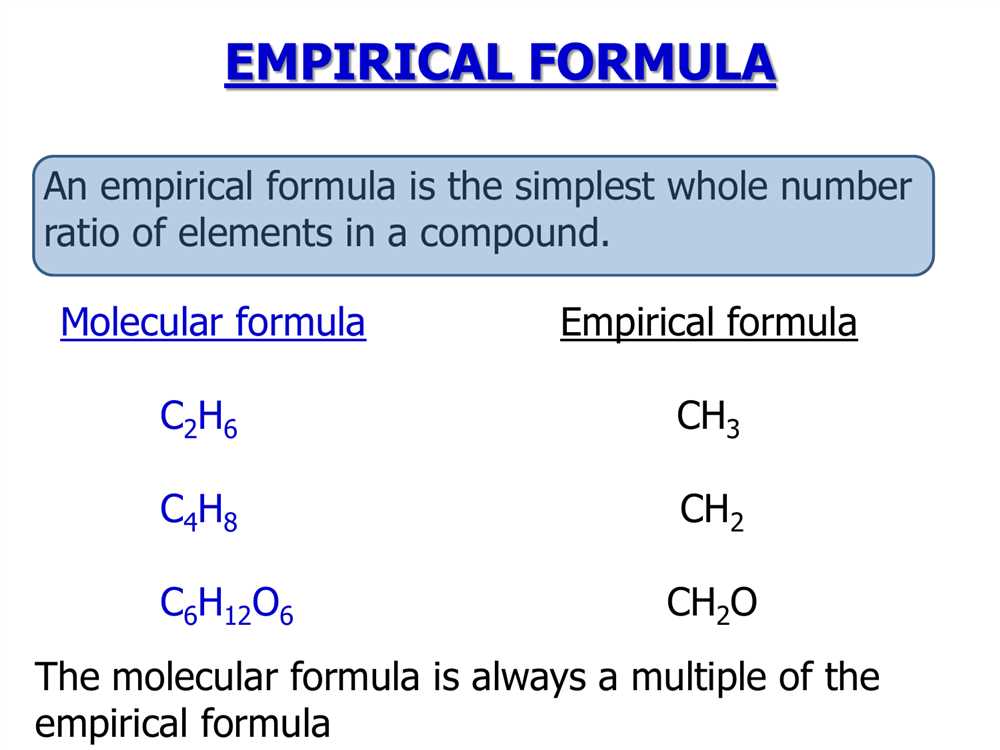
In the field of chemistry, empirical formulas play a crucial role in understanding the composition of compounds. Empirical formulas provide the simplest ratio of elements present in a compound, allowing chemists to identify the key elements and determine the overall structure of the compound.
One of the primary uses of empirical formulas is in determining the molecular formula of a compound. By knowing the empirical formula, chemists can then calculate the molecular formula by determining the actual number of atoms of each element in the compound. This information is essential in understanding the properties and behavior of the compound.
Empirical formulas also help in identifying unknown compounds. By analyzing the elemental composition of a compound, chemists can compare it to known empirical formulas and make educated guesses about the identity of the compound. This is particularly useful in forensic chemistry and analytical chemistry, where the identification of unknown substances is critical.
In addition, empirical formulas aid in stoichiometric calculations. Stoichiometry is the calculation of the quantities of reactants and products involved in a chemical reaction. By using empirical formulas, chemists can determine the amount of each element present in a compound, which is essential in balancing chemical equations and predicting the outcome of reactions.
- Empirical formulas also help in determining the percent composition of a compound. The percent composition refers to the relative mass of each element in a compound. By knowing the empirical formula and the atomic masses of the elements, chemists can calculate the percent composition and gain insights into the compound’s properties.
- Empirical formulas are widely used in organic chemistry. Organic compounds often have complex structures, and empirical formulas provide a simplified representation of their composition. This aids in classifying and naming organic compounds, as well as understanding their reactivity and behavior.
- Lastly, empirical formulas are essential in research and development. By understanding the empirical formula of a compound, researchers can make predictions about its properties and explore potential applications. This knowledge is instrumental in fields such as pharmaceuticals, materials science, and environmental science.
In conclusion, empirical formulas are a fundamental tool in chemistry that helps chemists understand the composition, structure, and behavior of compounds. From identifying unknown substances to predicting reaction outcomes, empirical formulas play a vital role in various areas of chemical research and application.
How to Determine the Empirical Formula of a Compound
When working with chemical compounds, it is important to know their empirical formula, which represents the simplest ratio of atoms in the compound. Determining the empirical formula allows scientists to understand the composition and properties of the compound. Here are the steps to determine the empirical formula of a compound:
Step 1: Collect the Mass or Percentage Composition Data
One of the first steps in determining the empirical formula is to gather data on the mass or percentage composition of the elements present in the compound. This information can be obtained through experiments or from other reliable sources.
Step 2: Convert Mass to Moles
Next, convert the mass of each element in the compound to moles. This can be done by dividing the mass of the element by its molar mass, which is the atomic weight of the element.
Step 3: Determine the Smallest Whole Number Ratio
After obtaining the moles of each element, determine the smallest whole number ratio by dividing each element’s moles by the smallest number of moles obtained. This will give you the subscripts of the elements in the empirical formula.
Step 4: Write the Empirical Formula
Finally, write the empirical formula using the subscripts obtained in the previous step. The subscripts indicate the number of atoms of each element in one molecule of the compound. The empirical formula is written in its simplest form.
By following these steps, scientists can determine the empirical formula of a compound, allowing them to understand its chemical composition and properties. This information is crucial in various scientific fields, including chemistry, biochemistry, and materials science.
The Mystery Gang’s Investigation
The investigation began with a thorough examination of the crime scene. The gang carefully collected samples of the unknown compound and analyzed its physical properties. They noted its color, odor, and texture, making detailed observations to aid in their investigation. Using their extensive knowledge of chemistry, they then performed various tests on the sample in order to determine its empirical formula.
First, Fred and Shaggy conducted a combustion analysis. They burned a small amount of the compound in the presence of excess oxygen and collected the gases produced. By measuring the mass of the carbon dioxide and water vapor formed, they were able to calculate the percentage composition of carbon and hydrogen in the compound.
Next, Velma and Daphne performed a titration to determine the compound’s molar mass. They dissolved a measured amount of the compound in water and added a standardized solution of an acid or base to neutralize the compound. By carefully measuring the volume of the standardized solution required to reach the equivalence point, they could calculate the molar mass of the compound.
Combining the results from both experiments, the gang was able to identify the compound’s empirical formula. They discovered that the compound contained carbon, hydrogen, and oxygen in a specific ratio. With this information, they were one step closer to solving the mystery at hand.
As the investigation progressed, the gang also interviewed witnesses, gathered additional evidence, and used their deductive reasoning skills to piece together the puzzle. With their collective efforts and scientific expertise, the Mystery Gang was determined to solve the case and bring justice to the culprit behind the mysterious compound.
The Empirical Formula of the Mystery Substance
After conducting a series of experiments and analysis, the Mystery Gang has successfully determined the empirical formula of the mysterious substance they encountered. Utilizing various techniques, such as elemental analysis and molecular weight determination, the team has been able to piece together the chemical composition of the substance.
The initial step in unveiling the empirical formula involved collecting a sample of the mystery substance and subjecting it to elemental analysis. This process allowed the team to identify the different elements present in the substance. By comparing the ratio of these elements, the gang was able to gain insight into the potential formula.
Next, the team focused on determining the molecular weight of the substance. Using techniques such as mass spectrometry and molecular formula calculations, they were able to obtain an accurate weight. This information, combined with the elemental analysis results, gave them further clues to narrow down the empirical formula.
Based on their findings, the Mystery Gang concluded that the empirical formula of the substance is C4H8O2. This means that the substance contains four carbon atoms, eight hydrogen atoms, and two oxygen atoms per molecule. With this newfound knowledge, the team is one step closer to understanding the nature and properties of the mystery substance.
Comparing the Mystery Substance’s Empirical Formula to Known Compounds
The first step in determining the nature of the mystery substance is to compare its empirical formula to known compounds. By analyzing the ratio of elements present in the empirical formula, scientists can make educated guesses about the possible identity of the substance. In this case, the empirical formula for the mystery substance is given as C6H6O2.
One way to compare the empirical formula is to look for compounds with similar ratios of elements. In this case, the ratio of carbon to hydrogen is 1:1, which is the same as in benzene (C6H6). However, the presence of an additional oxygen atom in the mystery substance sets it apart from benzene. This suggests that the mystery substance may be a derivative or derivative of benzene.
To further narrow down the possibilities, scientists can examine the properties and characteristics of compounds with similar empirical formulas. They can also consider any additional information or clues that may be available about the mystery substance. For example, if the substance is known to have a certain color, odor, or solubility, these characteristics could help in identifying potential compounds.
Another approach to comparing the empirical formula is to search databases or reference materials that catalog known compounds. By inputting the formula C6H6O2 into such a database, scientists can generate a list of compounds that match or closely resemble the mystery substance’s empirical formula. They can then examine the properties and characteristics of these compounds to see if any match the known properties of the mystery substance.
In conclusion, comparing the mystery substance’s empirical formula to known compounds is a crucial step in identifying its nature. By analyzing the ratio of elements and considering the properties and characteristics of compounds with similar formulas, scientists can make informed guesses about the identity of the mystery substance. Further experiments and analysis may be necessary to confirm these guesses and determine the exact composition of the substance.