
The periodic table is a fundamental tool in chemistry, allowing scientists to organize and understand the properties of all known elements. But what if there was a mystery periodic table with its own unique key? Imagine a hidden code within the table that unlocked a wealth of knowledge and discoveries. This article delves into the concept of a mystery periodic table answer key, exploring the potential secrets it could hold and the implications it might have for scientific understanding.
In this hypothetical scenario, the mystery periodic table answer key would be a set of hidden rules or patterns within the arrangement of elements on the table. These rules could reveal elusive connections and predict properties or behaviors that have eluded scientists thus far. It would be a key that unlocks the doors to new chemical reactions, structural arrangements, or even undiscovered elements.
One possible application of the mystery periodic table answer key could be the discovery of novel materials with extraordinary properties. Imagine a new metal that combines the strength of titanium with the electrical conductivity of copper, or a composite material that can withstand extreme temperatures without losing its structural integrity. With the help of the answer key, scientists would be able to predict the arrangement and properties of such materials, revolutionizing fields like engineering and manufacturing.
Furthermore, the mystery periodic table answer key might hold the secrets to unlocking the mysteries of the universe. By deciphering the hidden patterns within the table, scientists could gain insights into the fundamental forces and interactions that govern our world. This could lead to advancements in fields like quantum physics, cosmology, and even the search for extraterrestrial life.
Mystery Periodic Table Answer Key
Are you ready to solve the mystery of the periodic table? Look no further! Below you will find the answer key to all the elements in the mystery periodic table. But remember, before you uncover the answers, try to test your knowledge and see how many elements you can identify on your own!
The mystery periodic table included the following elements:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Beryllium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Fluorine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulfur (S)
- Chlorine (Cl)
- Potassium (K)
- Argon (Ar)
- Calcium (Ca)
These are just a few of the elements that make up the periodic table. Each element has its own unique properties and characteristics, and they all play a vital role in our world. By understanding the elements and their relationships, scientists are able to unlock the mysteries of the universe and make important discoveries. So, go ahead and explore the periodic table further to expand your knowledge and curiosity about the elements that shape our world!
What is the Mystery Periodic Table?
The Mystery Periodic Table is a educational tool designed to engage students in the study of the periodic table. It presents the chemical elements in a unique and interactive way, challenging students to solve puzzles and uncover hidden clues to unlock the secrets of the elements. Through this engaging approach, students not only learn the properties and characteristics of the elements, but also develop critical thinking and problem-solving skills.
Each element in the Mystery Periodic Table is associated with a mystery or puzzle that students must solve. The table is divided into sections, with each section containing a set of elements that share a common theme. As students explore each section, they will encounter various challenges, such as deciphering codes, solving riddles, or unraveling hidden messages. By successfully solving these challenges, students can progress through the table and unlock new elements and mysteries.
The Mystery Periodic Table offers an interactive and immersive learning experience that goes beyond traditional methods of teaching the periodic table. It encourages active participation and engagement, allowing students to become detectives and scientists as they search for answers and discover the fascinating world of chemistry. By incorporating elements of mystery and problem-solving, this unique approach to teaching the periodic table aims to make learning an enjoyable and rewarding experience for students.
Understanding the Structure of the Mystery Periodic Table
The Mystery Periodic Table is a unique and complex representation of the chemical elements. It is designed to challenge and engage students in their understanding of the periodic table and its patterns. By deciphering the structure of the Mystery Periodic Table, students can deepen their knowledge of the elements and their properties.
The Mystery Periodic Table is made up of various symbols, colors, and patterns that hold key information about the elements. Each element is represented by a distinct symbol, which can be identified by referring to the answer key. The colors and patterns on the table indicate different characteristics and trends of the elements, such as their atomic mass, electronegativity, or chemical reactivity.
- Atomic Mass: The atomic mass of each element is represented by a specific color or pattern on the Mystery Periodic Table. By analyzing the table, students can identify the elements with higher or lower atomic mass and observe any patterns or trends that may exist.
- Electronegativity: Electronegativity is another important property of the elements that can be understood through the Mystery Periodic Table. Elements with high electronegativity are indicated by a particular color or symbol, allowing students to recognize which elements are more likely to attract electrons.
- Chemical Reactivity: The Mystery Periodic Table also provides clues about the chemical reactivity of the elements. By examining the table, students can note any patterns in reactivity and make predictions about how different elements might interact with each other.
Through careful examination and analysis of the symbols, colors, and patterns on the Mystery Periodic Table, students can unlock the secrets hidden within and gain a deeper understanding of the elements and their properties. This interactive approach to learning the periodic table encourages critical thinking, problem-solving, and a holistic understanding of chemistry.
Exploring the Elements in the Mystery Periodic Table
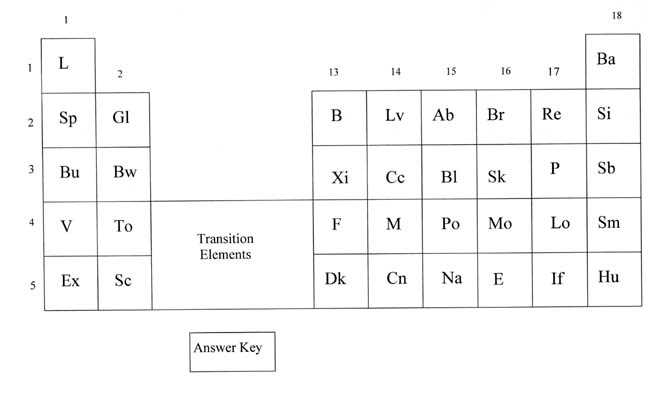
The mystery periodic table is an intriguing puzzle that sparks curiosity and challenges students to investigate and uncover the hidden elements. By using critical thinking skills and scientific knowledge, students can decipher the clues and solve the mystery of the unknown elements.
Each element in the mystery periodic table is represented by a symbol and a number. The symbols follow the standard naming conventions, but the numbers are replaced with question marks, leaving students to speculate on the atomic number of each element. Through careful analysis and comparison with known elements, students can make educated guesses about the missing information.
Symbol Clues:
The symbols themselves offer clues to the identity of the elements. For example, the symbol “Fe” is widely recognized as the symbol for iron. By association, students might infer that other symbols containing “Fe” could also represent transition metals. Similarly, symbols featuring “O” could indicate elements that are likely to be oxygen or oxygen-containing compounds.
Pattern Clues:
Another clue to solving the mystery is to observe patterns within the periodic table. By looking at the arrangement of elements, students may notice similarities and trends that can guide their deductions. For example, if a certain row or column in the periodic table is already filled with known elements, it is likely that the mystery elements will conform to the same pattern.
Comparative Analysis:
By comparing the properties and characteristics of known elements with those of the mystery elements, students can make educated predictions. For example, if an element has a similar reactivity to another known element, it may have a comparable position in the periodic table. By examining similarities and differences, students can narrow down the possibilities and make informed conclusions about the unknown elements.
To further assist students in their investigation, an answer key may be provided to verify their findings. This allows them to check their work, compare their deductions, and refine their understanding of the elements in the mystery periodic table. Through this process of exploration and analysis, students can develop a deeper understanding of the periodic table and the properties of the elements it contains.
Decoding the Symbols and Atomic Numbers
The periodic table is a fundamental tool in chemistry, allowing scientists to classify and understand the properties of various elements. One of the most basic pieces of information provided by the periodic table is the atomic number of each element, which represents the number of protons in the nucleus of an atom. Understanding the atomic numbers is crucial for deciphering the symbols used to represent each element.
The symbols on the periodic table are abbreviations for the names of the elements. These symbols are typically derived from the names of the elements, but not always in an intuitive way. For example, the symbol for hydrogen is H, which corresponds to the first letter of its name. However, some symbols may be derived from different languages or historical origins. The symbol for gold, Au, comes from the Latin word for gold, “aurum”. These symbols are standardized and globally recognized by chemists.
To further understand the meaning behind the symbols, it is important to analyze the atomic numbers. The atomic number provides information about an element’s identity within the periodic table. Elements with the same atomic number have the same number of protons, making them chemically similar. For example, all elements with an atomic number of 6 are classified as carbon, while those with an atomic number of 8 are classified as oxygen. Atomic numbers also determine the placement of elements in the periodic table, as they increase from left to right and from top to bottom.
Decoding the symbols and atomic numbers of the periodic table is essential for understanding the properties and behaviors of elements. By analyzing these codes, scientists can predict how different elements will interact and react with each other, allowing them to make breakthroughs in various fields such as medicine, technology, and materials science.
The Significance of the Mystery Periodic Table
The Mystery Periodic Table is an intriguing and puzzling tool that presents a unique challenge for scientists and chemists. With its unconventional symbols and unknown elements, it sparks curiosity and pushes the boundaries of our understanding of the periodic table. The significance of this mysterious table lies in its potential to unlock new discoveries and expand our knowledge of the elements and their properties.
One key aspect of the Mystery Periodic Table is its ability to engage students and enthusiasts in the field of chemistry. By presenting a puzzle for them to solve, it encourages critical thinking, problem-solving skills, and the development of scientific inquiry. It fosters a sense of excitement and adventure, as individuals strive to unravel the secrets hidden within the table.
The Mystery Periodic Table also holds the promise of new discoveries and breakthroughs in the field of chemistry. By challenging established norms and conventions, it pushes scientists to think outside the box and explore new possibilities. The unknown elements and symbols on the table could represent undiscovered elements or variations of existing ones, which may have unique properties and applications.
Furthermore, the Mystery Periodic Table serves as a reminder of the vastness and complexity of the natural world. It highlights the fact that there is still so much we don’t know and understand about the elements and their behavior. It encourages scientists to continue their exploration and research, driving us towards a deeper understanding of the fundamental building blocks of our universe.
In conclusion, the Mystery Periodic Table is not just a mere curiosity, but a valuable tool for scientific exploration and discovery. Its significance lies in its ability to engage, challenge, and inspire scientists and enthusiasts alike. With its potential to unlock new knowledge and push the boundaries of our understanding, it serves as a testament to the never-ending quest for knowledge in the field of chemistry.
Unraveling the Patterns and Trends

The periodic table is a powerful tool that allows scientists to organize and understand the elements. It provides a systematic arrangement of all the known elements based on their chemical properties and atomic numbers. By studying the periodic table, scientists are able to identify patterns and trends that help them predict the behavior of elements and discover new ones.
One of the most important patterns in the periodic table is the periodicity of elemental properties. Elements in the same group or column tend to have similar chemical properties, while elements in the same period or row often exhibit similar physical properties. This pattern allows scientists to make predictions about the behavior of unidentified elements based on their position in the table.
The periodic table also reveals trends in atomic size, electronegativity, ionization energy, and other important properties of elements. For example, as you move from left to right across a period, the atomic size generally decreases while electronegativity increases. This trend can be explained by the increasing number of protons in the nucleus, which attracts the electrons more strongly. Similarly, as you move down a group, the atomic size generally increases while electronegativity decreases.
Other trends observed in the periodic table include the reactivity of metals and nonmetals, the formation of positive and negative ions, and the stability of different oxidation states. By studying these patterns and trends, scientists can make informed predictions about the behavior of elements and use this knowledge to design new materials and compounds with specific properties. The periodic table truly serves as a roadmap for unraveling the mysteries of the elements and understanding the fundamental building blocks of the universe.