
If you are currently working on a naming acids worksheet and need answers, you have come to the right place. Naming acids can be a challenging task, but with the right guidance and practice, you can become proficient in this skill. In this article, we will provide you with the answers to a naming acids worksheet, allowing you to check your work and understand the correct naming conventions.
Acids are compounds that can donate protons (H+) in a chemical reaction. When naming acids, it is important to consider the presence of hydrogen and the type of non-metal element present in the compound. The naming of acids often involves the use of prefixes and suffixes to indicate the number of hydrogen atoms present and the type of non-metal element.
In the naming acids worksheet, you might encounter different types of acids, such as binary acids and oxyacids. Binary acids consist of hydrogen and a non-metal element, while oxyacids contain hydrogen, oxygen, and a non-metal element. Each type of acid has its own naming conventions, and understanding these rules is crucial for accurately identifying and naming acids.
Naming Acids Worksheet Answers
Below are the correct answers for the naming acids worksheet:
1. HCl – hydrochloric acid
Hydrochloric acid is a strong acid that is commonly found in the stomach and has a chemical formula of HCl. It is used in many industrial processes and is also an important component in the production of hydrochloric acid.
2. HNO3 – nitric acid
Nitric acid is a strong acid that is used in the production of fertilizers, explosives, and dyes. It has a chemical formula of HNO3 and is highly corrosive.
3. H2SO4 – sulfuric acid
Sulfuric acid is a strong acid that is widely used in the chemical industry. It has a chemical formula of H2SO4 and is known for its corrosive and oxidizing properties.
4. H3PO4 – phosphoric acid
Phosphoric acid is a weak acid that is commonly used as a food additive, particularly in soft drinks. It has a chemical formula of H3PO4 and is also used in the production of fertilizers and detergents.
5. H2CO3 – carbonic acid
Carbonic acid is a weak acid that is formed when carbon dioxide dissolves in water. It has a chemical formula of H2CO3 and is responsible for the fizz and tangy taste in carbonated beverages.
6. H3BO3 – boric acid
Boric acid is a weak acid that is commonly used as an antiseptic and insecticide. It has a chemical formula of H3BO3 and is also used in the production of ceramics and glass.
- Overall, naming acids requires an understanding of the rules for naming compounds and knowing the names and formulas of common acids.
- Remember that the prefix “hydro-” is used for binary acids (hydrogen + nonmetal), while the prefix “per-” is used for acids containing a greater number of oxygen atoms and the prefix “hypo-” is used for acids containing a smaller number of oxygen atoms.
- The suffix “-ic” is used for the acid with the higher oxidation state, while the suffix “-ous” is used for the acid with the lower oxidation state.
By practicing naming acids and understanding their properties, you can gain a better understanding of their role in chemistry and various applications.
Naming Binary Acids
The naming of binary acids follows a specific set of rules to ensure consistency and clarity. Binary acids are compounds that contain hydrogen and a nonmetal. When naming binary acids, the hydrogen is always listed first, followed by the nonmetal. The name of the nonmetal is modified to indicate that it is an acid.
To name a binary acid, the prefix “hydro-” is added to the name of the nonmetal, followed by the suffix “-ic”. For example, the binary acid composed of hydrogen and chlorine is called hydrochloric acid. Similarly, the binary acid composed of hydrogen and sulfur is called hydrosulfuric acid.
It is important to note that binary acids do not contain oxygen. Acids that contain oxygen are named differently and fall under the category of oxyacids. Oxyacids have their own set of rules for naming based on the presence and oxidation state of oxygen in the compound.
Overall, the naming of binary acids is relatively straightforward and relies on the use of specific prefixes and suffixes to indicate the presence of hydrogen and the acidic nature of the compound. Understanding these rules can aid in the identification and naming of binary acids in various chemical contexts.
Naming Oxyacids
Oxyacids are a type of acid that contain oxygen atoms. They are composed of hydrogen, oxygen, and one or more additional elements. Oxyacids are generally formed when a non-metal element combines with oxygen and hydrogen. These acids have specific naming rules based on the central non-metal element and the number of oxygen atoms in the compound.
When naming oxyacids, the first step is to identify the central non-metal element. This element is named using its element name, followed by the word “acid”. For example, if the central non-metal element is sulfur, the acid would be named “sulfuric acid”.
The second step in naming oxyacids is determining the number of oxygen atoms present in the compound. Oxyacids with the most oxygen atoms have the suffix “-ic” in their name. For example, if the central non-metal element is chlorine and there are four oxygen atoms, the acid would be named “perchloric acid”.
If the oxyacid has one fewer oxygen atom than the “-ic” acid, it is given the suffix “-ous” in its name. For example, if the central non-metal element is nitrogen and there are three oxygen atoms, the acid would be named “nitrous acid”.
If the oxyacid has two fewer oxygen atoms than the “-ic” acid, it is given the prefix “hypo-” and the suffix “-ous” in its name. For example, if the central non-metal element is hypophosphorus and there is one oxygen atom, the acid would be named “hypophosphorous acid”.
In summary, naming oxyacids involves identifying the central non-metal element and determining the number of oxygen atoms present in the compound. By following the specific naming rules, oxyacids can be named accurately and systematically.
Naming Acids with Polyatomic Ions
When naming acids that contain polyatomic ions, there are specific naming rules that need to be followed. These rules help to determine the correct name of the acid based on the polyatomic ion it contains. Here are some examples of how to name acids with polyatomic ions:
1. Naming acids with -ate polyatomic ions: When the polyatomic ion ends in -ate, the suffix -ic is added to the name of the ion. For example, the polyatomic ion sulfate becomes sulfuric acid, and the polyatomic ion phosphate becomes phosphoric acid.
2. Naming acids with -ite polyatomic ions: When the polyatomic ion ends in -ite, the suffix -ous is added to the name of the ion. For example, the polyatomic ion sulfite becomes sulfurous acid, and the polyatomic ion nitrite becomes nitrous acid.
These naming rules ensure that the name of the acid reflects the composition of the polyatomic ion it contains. By following these rules, chemists can accurately name and identify acids containing polyatomic ions.
- Example:
- Polyatomic ion: Carbonate
- Acidic name: Carbonic acid
| Polyatomic Ion | Acidic Name |
|---|---|
| Sulfate | Sulfuric acid |
| Phosphate | Phosphoric acid |
| Nitrite | Nitrous acid |
Naming Acids with Transition Metals
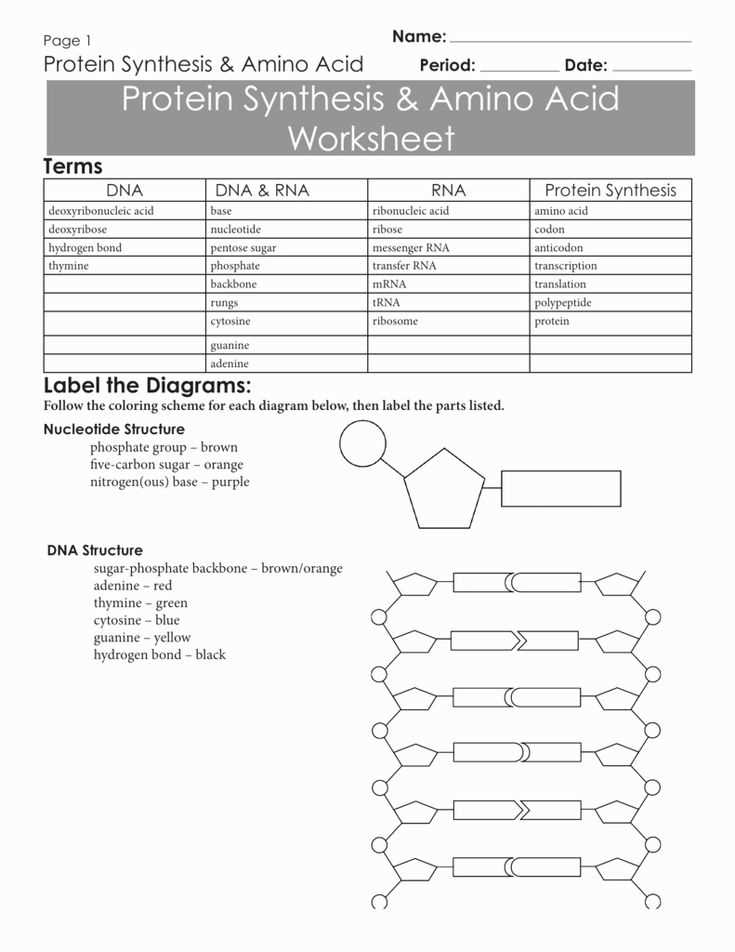
Transition metals are a group of elements in the periodic table that have unique properties and can form different types of compounds, including acids. Naming acids with transition metals follows specific rules to indicate their composition. These rules are based on the oxidation state of the metal and the type of acid being formed.
One common type of acid formed with transition metals is the binary acid, which contains hydrogen and a non-metal. These acids are named by adding the prefix “hydro-” to the root name of the non-metal, followed by the suffix “-ic” and the word “acid.” For example, if the transition metal is iron and it forms an acid with chlorine, the acid would be named hydrochloric acid.
Another type of acid formed with transition metals is the oxyacid, which contains hydrogen, oxygen, and a non-metal. These acids are named based on the oxidation state of the metal. If the metal has a lower oxidation state, the acid is named using the root name of the non-metal with the suffix “-ous” and the word “acid.” If the metal has a higher oxidation state, the acid is named using the root name of the non-metal with the suffix “-ic” and the word “acid.” For example, if the transition metal is chromium and it forms an acid with sulfur, the acid with the lower oxidation state would be named sulfurous acid, and the acid with the higher oxidation state would be named sulfuric acid.
In conclusion, naming acids with transition metals follows specific rules based on the type of acid and the oxidation state of the metal. These rules ensure consistent and accurate naming of acids within the field of chemistry.
Practice Problems: Naming Acids
In chemistry, acids are substances that release hydrogen ions (H+) when dissolved in water. Naming acids can be a challenging task as it involves understanding the chemical formulas and their corresponding rules. Here are some practice problems to help you master the skill of naming acids:
Problem 1:

What is the name of the acid with the formula HCl?
Solution: The formula HCl represents hydrogen chloride. To name this acid, we replace the -ide suffix with -ic acid. Therefore, the name of the acid with the formula HCl is hydrochloric acid.
Problem 2:
What is the name of the acid with the formula H2SO4?
Solution: The formula H2SO4 represents sulfuric acid. Since sulfur is a nonmetal, we do not change the ending of the name. Therefore, the name of the acid with the formula H2SO4 is sulfuric acid.
Problem 3:
What is the name of the acid with the formula H3PO3?
Solution: The formula H3PO3 represents phosphorous acid. Since phosphorous has a lower oxidation state, we use the -ous suffix. Therefore, the name of the acid with the formula H3PO3 is phosphorous acid.
Problem 4:
What is the name of the acid with the formula HNO2?
Solution: The formula HNO2 represents nitrous acid. Similar to phosphorous acid, we use the -ous suffix for lower oxidation states. Therefore, the name of the acid with the formula HNO2 is nitrous acid.
By practicing naming acids, you will become more familiar with the different rules and patterns. It is important to understand the nomenclature of acids as they play a crucial role in various chemical reactions and equations.
Answer Key: Naming Acids Worksheet

In chemistry, acids are compounds that release hydrogen ions (H+) when dissolved in water. They have distinct naming conventions based on their molecular composition and the number of oxygen atoms present. This answer key will help you correctly identify and name different types of acids.
1. Binary Acids
Binary acids are composed of hydrogen and a nonmetal element. To name a binary acid, use the prefix “hydro-” followed by the root name of the nonmetal element with the suffix “-ic” and the word “acid”. For example, HCl is hydrochloric acid.
2. Oxyacids
Oxyacids are acids that contain hydrogen, oxygen, and a nonmetal element. The naming of oxyacids depends on the number of oxygen atoms in the molecule. If the nonmetal element ends in “-ate”, change the ending to “-ic” and add the word “acid”. For example, HNO3 becomes nitric acid. If the nonmetal element ends in “-ite”, change the ending to “-ous” and add the word “acid”. For example, HNO2 becomes nitrous acid.
3. Acid Prefixes
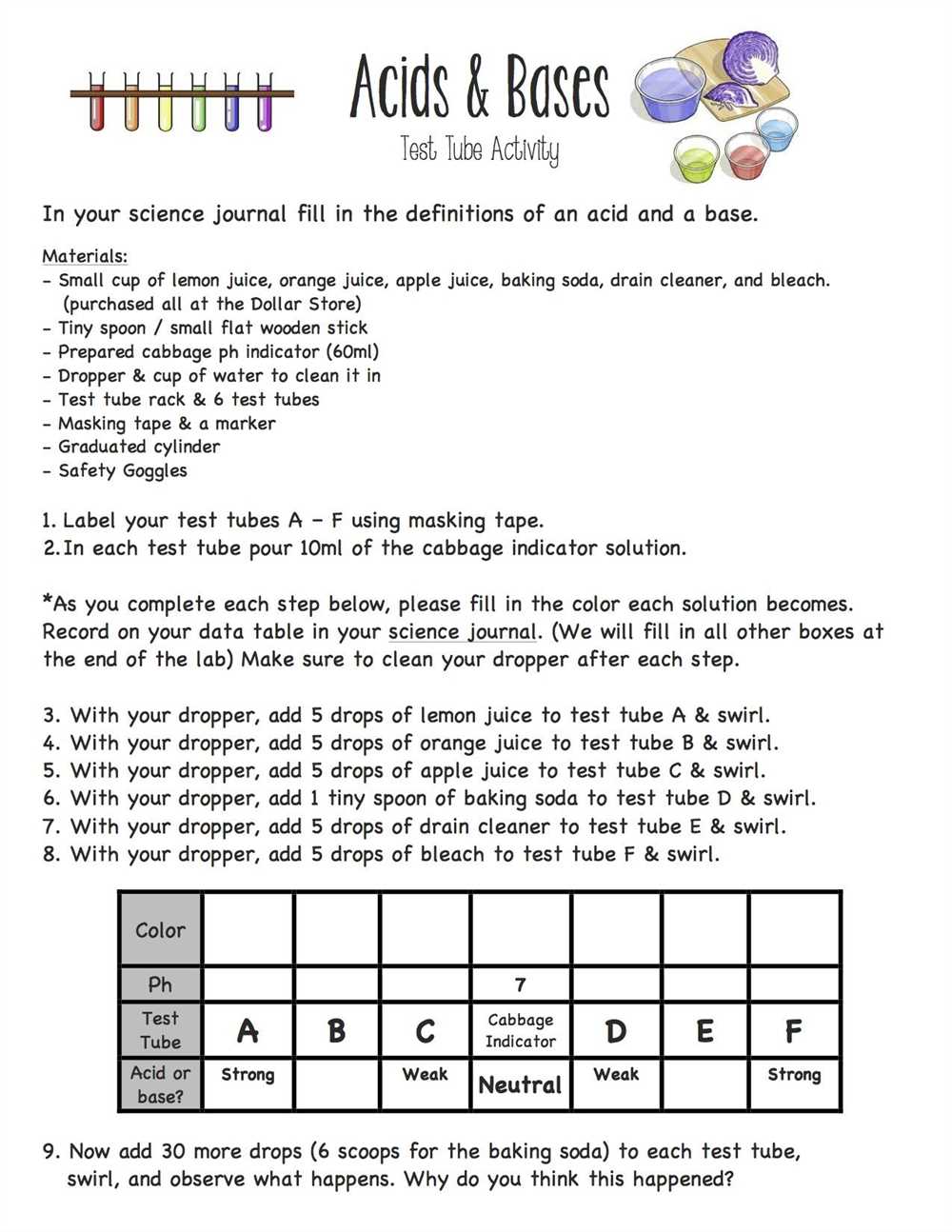
Some acids contain additional prefixes to indicate the number of oxygen atoms. For example, H2CO3 is carbonic acid because it contains the prefix “carbon-” to indicate the presence of two oxygen atoms. Similarly, HClO3 is chloric acid because it contains the prefix “chlor-” to indicate the presence of three oxygen atoms.
Overall, naming acids involves understanding the composition of the acid molecule and applying the appropriate naming conventions. This answer key provides examples and explanations to help you correctly name different types of acids. Practice and familiarity with these naming rules will help you become proficient in naming acids.
Additional Resources for Naming Acids
If you are looking for additional resources to help you practice naming acids, there are many options available online. These resources can provide you with extra practice problems and explanations to further solidify your understanding of acid naming rules.
1. Interactive Online Worksheets: Some websites offer interactive worksheets that allow you to practice naming acids and receive instant feedback on your answers. These worksheets often include a variety of acid naming problems, ranging from simple to more complex. You can complete them at your own pace and receive immediate feedback on your progress.
2. Video Tutorials: Video tutorials are another useful resource for learning how to name acids. Many educational websites and online platforms offer video lessons specifically focused on acid naming. These videos often provide step-by-step explanations and examples to help you understand the naming rules and apply them to different acid compounds.
3. Flashcards: Flashcards can be a helpful tool to reinforce your knowledge of acid naming. Creating your own flashcards or using pre-made ones can help you memorize the names and formulas of different acids. By regularly reviewing these flashcards, you can improve your ability to quickly identify and name acids.
4. Practice Worksheets and Quizzes: Numerous websites offer printable practice worksheets and quizzes on acid naming. These resources typically provide a range of acid naming problems for you to solve. Working through these worksheets and quizzes can help you gain confidence and improve your skills in naming acids.
5. Online Forums and Discussion Boards: If you have specific questions or need further clarification on acid naming, online forums and discussion boards can be a valuable resource. You can post your questions and receive answers from experts or other students. These platforms also provide an opportunity to engage in discussions and share tips and strategies with others who are learning to name acids.
By utilizing these additional resources, you can enhance your understanding of acid naming and become more proficient in identifying and naming different types of acids. Remember to practice regularly and seek help whenever you need it to ensure mastery of this important chemistry skill.