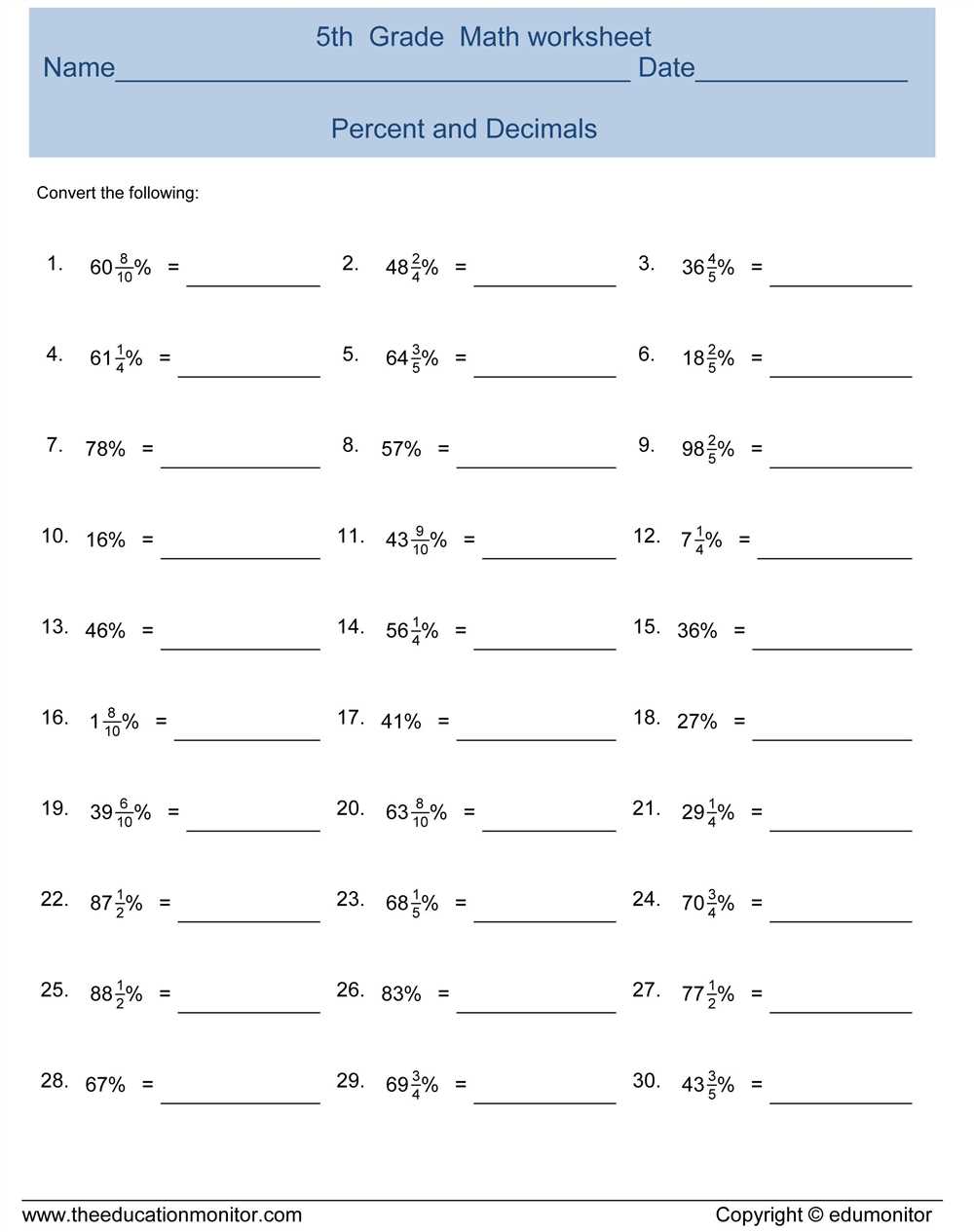
Understanding percent composition is crucial in chemistry as it helps determine the amount of each element present in a compound. To accurately calculate percent composition, students must be able to determine the molar masses of the elements and the overall compound. Practice worksheets are a great way for students to reinforce their understanding and gain confidence in performing these calculations.
By providing students with a percent composition practice worksheet, they are given the opportunity to apply the concepts they have learned in the classroom. These worksheets typically consist of various chemical formulas, for which students must calculate the percent composition of each element. The answers to these worksheets provide students with immediate feedback, allowing them to identify any mistakes and learn from them.
The percent composition practice worksheet answers act as a valuable resource for both students and teachers. Students can use them to check their work and ensure accuracy, while teachers can use them as a reference to assist students who may be struggling. They also serve as a guide for students to understand the correct method of calculation, as they can compare their answers to the provided solutions.
Overall, percent composition practice worksheet answers serve as a tool to enhance student learning and comprehension in chemistry. By actively engaging in these practice exercises, students can solidify their understanding of percent composition and gain the confidence needed to excel in future chemistry studies.
Understanding Percent Composition
Percent composition is a concept used in chemistry to determine the relative amounts of elements in a compound. It is expressed as the percentage of each element’s mass in relation to the total mass of the compound. Understanding percent composition is essential for various applications, such as predicting chemical reactions, determining empirical formulas, and analyzing chemical reactions.
Calculating Percent Composition: To calculate percent composition, you first need to know the formula of the compound and the atomic masses of the elements it contains. The steps involved in calculating percent composition are as follows:
- Determine the molar mass of the compound by adding up the atomic masses of all the elements in the formula.
- Divide the molar mass of each element by the molar mass of the compound.
- Multiply the result by 100 to obtain the percent composition of each element.
Example: Let’s take the compound H2O (water) as an example. The molar mass of water is determined by adding the atomic masses of hydrogen (H) and oxygen (O), which are approximately 1 g/mol and 16 g/mol, respectively. The molar mass of water is therefore 18 g/mol. To calculate the percent composition of hydrogen, divide the molar mass of hydrogen (2 g/mol) by the molar mass of water (18 g/mol) and multiply by 100. The percent composition of hydrogen in water is 11.11%. Similarly, the percent composition of oxygen in water is 88.89%.
Applications: Percent composition is used in various ways in chemistry. It is useful in determining the empirical formula of a compound, which represents the simplest whole number ratio of elements present. It is also helpful in predicting the products and quantities of reactants involved in chemical reactions. Additionally, percent composition is used in analyzing the purity of substances, as impurities can affect the percent composition of a compound.
In conclusion, understanding percent composition allows chemists to analyze and predict the behavior of compounds in various chemical reactions. It provides valuable information about the relative amounts of elements in a compound and is an essential concept in the field of chemistry.
What is Percent Composition?
Percent composition is a method used in chemistry to determine the relative abundance of each element in a compound. It is expressed as a percentage, indicating the proportion of each element’s mass in the total mass of the compound. This information is crucial for understanding the chemical properties and behavior of a substance.
To calculate the percent composition, the mass of each element in the compound is divided by the molar mass of the compound and multiplied by 100. The molar mass is the mass of one mole of the compound, which can be calculated by summing the atomic masses of all the atoms in the formula. The resulting percentage represents the proportion of that element in the compound.
Percent composition is particularly useful because it allows chemists to compare different compounds and assess their purity. By analyzing the percent composition, one can determine if a compound is contaminated with impurities or if it is a pure substance. This information is vital in various fields, including pharmaceuticals, environmental science, and materials science.
Moreover, percent composition can also be used to predict the properties and behavior of compounds. For example, knowing the percent composition of a compound can help determine its melting and boiling points, density, and reactivity. This knowledge is essential for designing and synthesizing new materials or understanding the behavior of known substances in different conditions.
In conclusion, percent composition is a fundamental concept in chemistry that enables scientists to determine the relative abundance of elements in a compound. It provides valuable information about the chemical properties, purity, and behavior of substances, making it an essential tool in various scientific fields.
Why is Percent Composition Important?
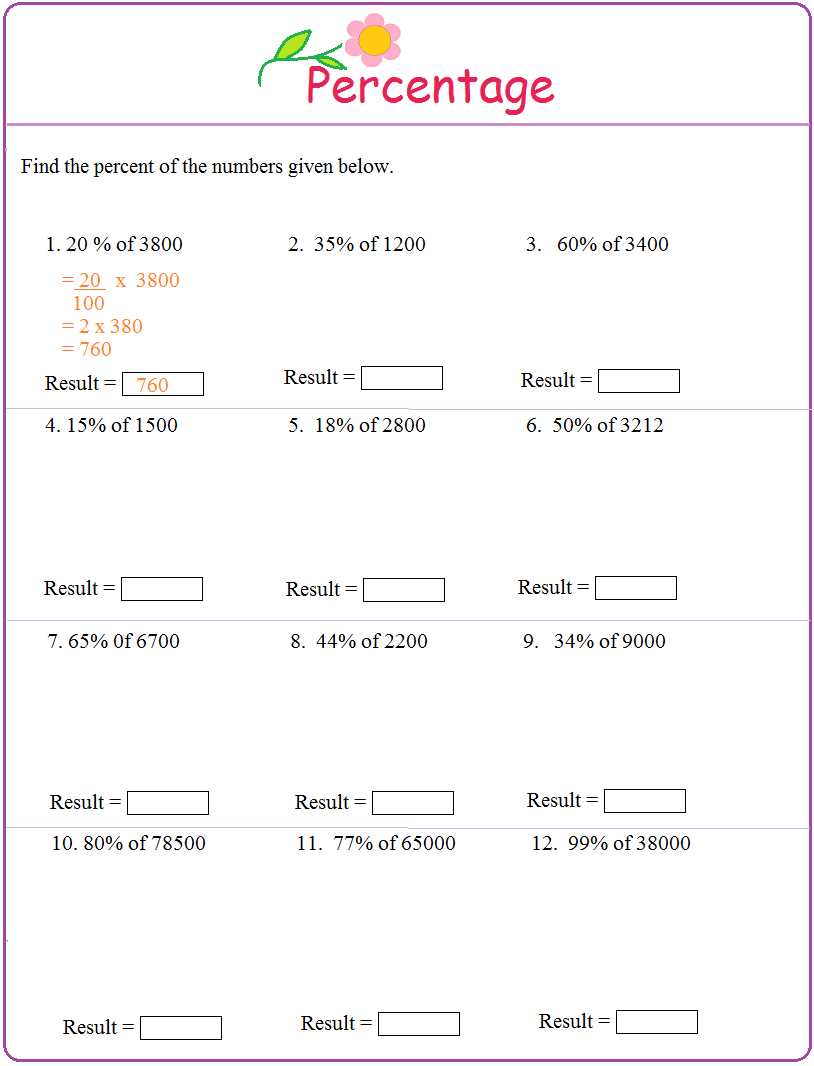
The concept of percent composition is an essential aspect of chemistry as it allows scientists to determine the proportion of each element present in a compound or mixture. By knowing the percent composition, scientists can analyze and understand the chemical properties, behavior, and reactivity of substances. This information is crucial for various fields of study such as materials science, pharmacology, and environmental chemistry.
1. Determining Chemical Formulas: Percent composition helps chemists determine the empirical and molecular formulas of compounds. By analyzing the percentages of each element in a compound, chemists can deduce the ratio of atoms and the overall chemical formula. This information is invaluable in understanding the structure and properties of substances.
2. Identifying Impurities and Purity: Knowing the percent composition allows chemists to identify impurities present in a substance. By comparing the expected composition with the observed composition, scientists can determine the purity of a compound. This knowledge is vital in manufacturing industries, quality control, and ensuring the safety and effectiveness of pharmaceutical drugs.
3. Balancing Chemical Equations: Percent composition plays a crucial role in balancing chemical equations. By understanding the compositions of reactants and products, chemists can balance equations by adjusting the mole ratios. This process is fundamental in chemical reactions and predicting the yield of products.
4. Predicting Physical and Chemical Properties: The percent composition provides valuable insights into the physical and chemical properties of substances. For example, the percent composition of a compound can determine its melting and boiling points, density, solubility, and reactivity. This information is essential in designing new materials, understanding their behavior, and developing applications in various industries.
5. Environmental and Toxicology Studies: Percent composition is crucial in environmental chemistry and toxicology studies. By determining the percentage of elements present in pollutants or toxic substances, scientists can assess their impact on the environment and human health. This data helps in developing strategies for pollution control, remediation, and risk assessment.
In conclusion, percent composition is of utmost importance in the field of chemistry as it provides crucial information about the composition, structure, and properties of substances. This knowledge has far-reaching implications in various scientific disciplines and practical applications, making percent composition an indispensable tool for chemists and researchers.
Calculation of Percent Composition
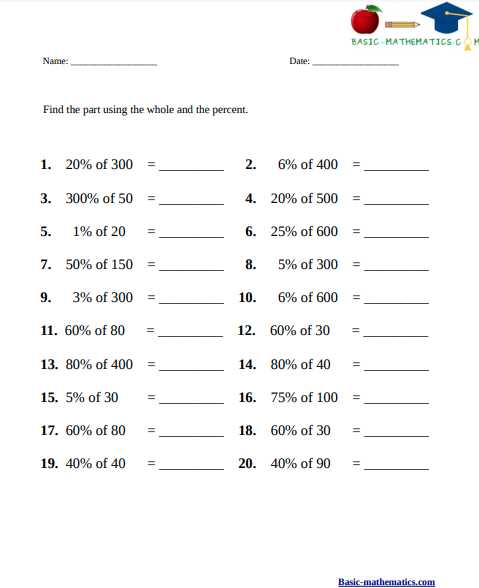
The percent composition of a compound refers to the percentage of each element present in the compound. It is calculated by dividing the molar mass of each element by the molar mass of the compound and then multiplying by 100. This allows us to understand the relative abundance of each element in a given substance.
To calculate the percent composition, we first need to determine the molar mass of the compound. This is done by adding up the atomic masses of each element present in the formula of the compound. We can find the atomic masses of elements from the periodic table.
Once we have the molar mass of the compound, we can then calculate the percent composition of each element. This is done by dividing the molar mass of the element by the molar mass of the compound and multiplying by 100. The result gives us the percentage of that particular element in the compound.
For example, let’s consider the compound water (H2O). The molar mass of water is calculated by adding the atomic masses of hydrogen (H) and oxygen (O) together. The atomic mass of hydrogen is approximately 1 gram per mole, and the atomic mass of oxygen is approximately 16 grams per mole. Therefore, the molar mass of water is approximately 18 grams per mole.
To calculate the percent composition of hydrogen in water, we divide the molar mass of hydrogen (2 grams per mole) by the molar mass of water (18 grams per mole) and multiply by 100. This gives us a percent composition of approximately 11.1%. Similarly, the percent composition of oxygen in water would be approximately 88.9%.
Overall, calculating the percent composition of a compound allows us to understand the elemental makeup of a substance and analyze its properties and behavior in various chemical reactions.
Formula for Percent Composition
The percent composition of a compound is a way to express the relative amounts of each element in the compound. It is calculated by dividing the mass of each element by the total mass of the compound, and then multiplying by 100 to get the percentage.
To calculate percent composition, you need to know the chemical formula of the compound and the atomic masses of the elements involved. The chemical formula tells you the number of atoms of each element present in one molecule of the compound. The atomic masses can be found on the periodic table.
Here is the formula for percent composition:
- Step 1: Calculate the molar mass of the compound by adding up the atomic masses of all the elements in the formula.
- Step 2: Calculate the mass of each element in the compound by multiplying the number of atoms of that element by its atomic mass.
- Step 3: Divide the mass of each element by the molar mass of the compound.
- Step 4: Multiply the result by 100 to get the percentage of each element.
For example, let’s calculate the percent composition of water (H2O).
- Step 1: The molar mass of water is calculated as (2 * atomic mass of hydrogen) + atomic mass of oxygen. Let’s assume the atomic mass of hydrogen is 1 g/mol and the atomic mass of oxygen is 16 g/mol. The molar mass of water is therefore 2 g/mol + 16 g/mol = 18 g/mol.
- Step 2: The mass of hydrogen in water is (2 * 1 g/mol) = 2 g. The mass of oxygen in water is 16 g.
- Step 3: The percent composition of hydrogen is (2 g / 18 g) * 100 = 11.11%. The percent composition of oxygen is (16 g / 18 g) * 100 = 88.88%.
By calculating the percent composition, we can better understand the chemical and physical properties of a compound and how its elements are arranged and interact within the structure.
Example Problem: Calculating Percent Composition
Let’s consider an example to understand how to calculate percent composition. We will determine the percent composition of carbon and hydrogen in a compound.
Suppose we have a compound with the formula C2H4. To calculate the percent composition, we need to find the molar mass of carbon and hydrogen, as well as the total molar mass of the compound. The molar mass of carbon is 12.01 g/mol and the molar mass of hydrogen is 1.01 g/mol.
First, we calculate the molar mass of carbon in the compound. Since there are 2 carbon atoms in C2H4, the molar mass of carbon is 2 * 12.01 g/mol = 24.02 g/mol.
Next, we calculate the molar mass of hydrogen in the compound. Since there are 4 hydrogen atoms in C2H4, the molar mass of hydrogen is 4 * 1.01 g/mol = 4.04 g/mol.
Now, we calculate the total molar mass of the compound. The total molar mass is the sum of the molar masses of carbon and hydrogen, which is 24.02 g/mol + 4.04 g/mol = 28.06 g/mol.
To determine the percent composition of carbon in the compound, we divide the molar mass of carbon by the total molar mass of the compound and multiply by 100. The percent composition of carbon is (24.02 g/mol / 28.06 g/mol) * 100% = 85.6%.
Similarly, to determine the percent composition of hydrogen in the compound, we divide the molar mass of hydrogen by the total molar mass of the compound and multiply by 100. The percent composition of hydrogen is (4.04 g/mol / 28.06 g/mol) * 100% = 14.4%.
Therefore, the percent composition of carbon in the compound C2H4 is 85.6% and the percent composition of hydrogen is 14.4%.
Comparison of Experimental and Theoretical Percent Composition
When conducting experiments in the field of chemistry, it is important to compare the experimental percent composition of a compound with its theoretical percent composition. The experimental percent composition is determined through laboratory experiments, while the theoretical percent composition is calculated based on the chemical formula of the compound and the atomic masses of its elements.
The experimental percent composition is obtained by measuring the masses of the different elements present in the compound and calculating their percentage by mass. This provides a real-world representation of the compound’s composition, taking into account any impurities or variations that may have occurred during the experiment. On the other hand, the theoretical percent composition is calculated using the formula of the compound and assumes that the compound is pure and all reactions have occurred as expected.
Comparing the experimental and theoretical percent compositions allows scientists to evaluate the accuracy and reliability of their experimental results. If the experimental percent composition is close to the theoretical percent composition, it indicates that the experiment was conducted accurately and the compound was synthesized correctly. However, if there is a significant difference between the two values, it may suggest errors in the experiment, such as incorrect measurements or impurities in the sample.
In addition to evaluating the accuracy of experimental results, comparing the experimental and theoretical percent compositions can also provide insights into the nature of the compound. If the experimental percent composition deviates significantly from the theoretical percent composition, it may suggest the presence of additional compounds or unexpected reactions that occurred during the experiment. This can lead to further investigations and discoveries in the field of chemistry.