
If you are a student of chemistry or simply have an interest in the elements, chances are you have come across the periodic table. This iconic chart organizes the elements based on their atomic number and other properties. However, understanding the periodic table can be challenging, and you may have questions about its structure and function.
In this article, we will provide you with a comprehensive list of frequently asked questions about the periodic table, along with their answers in a PDF format. Whether you want to know why the elements are arranged in a specific order or how to read the information presented on the table, this PDF will serve as a handy guide.
Inside the PDF, you will find explanations for concepts such as atomic number, atomic mass, period, group, and electron configuration. Additionally, you will learn about the significance of certain elements and their properties, and how they relate to each other on the periodic table. With this information at your disposal, you will develop a deeper understanding of the elements and their role in chemistry.
Overview of the periodic table
The periodic table is a systematic arrangement of chemical elements according to their atomic number, electron configuration, and recurring chemical properties. It is a fundamental tool in chemistry and provides a comprehensive overview of the building blocks of matter.
The periodic table is organized in rows called periods and columns called groups. Each period represents a higher energy level of electrons, while each group contains elements with similar chemical behavior. Elements in the same group have the same number of valence electrons, which determines their chemical reactivity.
The periodic table is divided into several blocks:
- The s-block consists of groups 1 and 2, as well as helium
- The p-block includes groups 13-18
- The d-block encompasses groups 3-12
- The f-block is located below the main body of the table and consists of the lanthanides and actinides
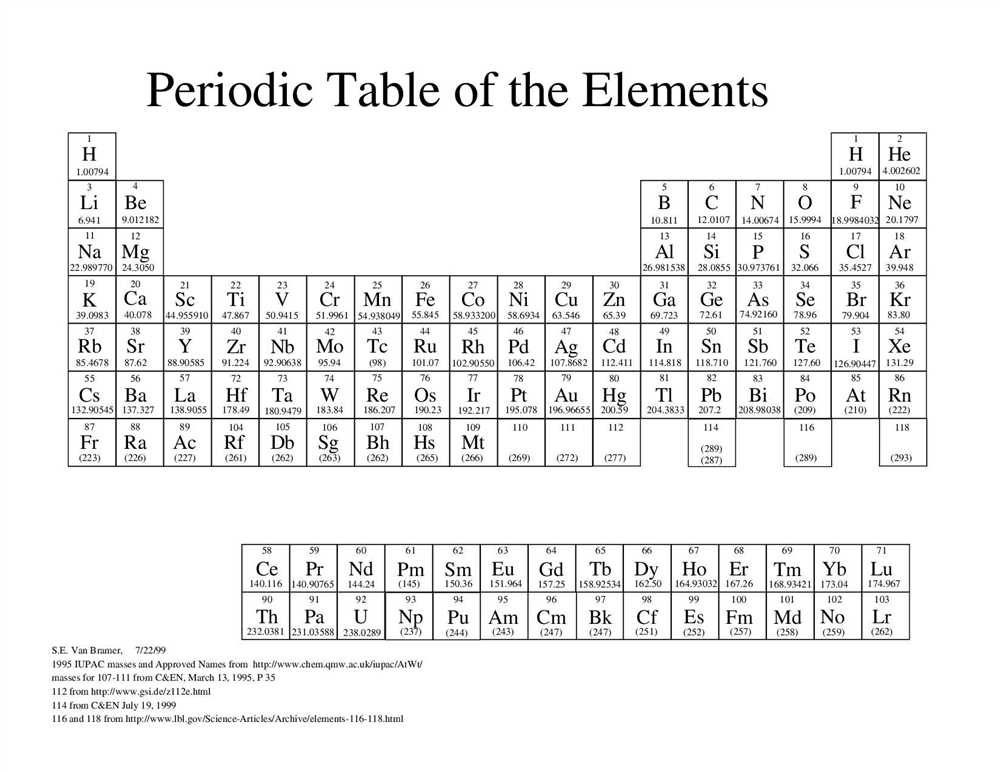
Elements within the periodic table are represented by their unique chemical symbol, atomic number, atomic mass, and electron configuration. Out of the known 118 elements, 92 occur naturally on Earth, while the rest are synthetic.
| Symbol | Name | Atomic Number | Atomic Mass |
|---|---|---|---|
| H | Hydrogen | 1 | 1.008 |
| He | Helium | 2 | 4.0026 |
| Li | Lithium | 3 | 6.94 |
| Be | Beryllium | 4 | 9.0122 |
| B | Boron | 5 | 10.81 |
Understanding the periodic table allows scientists to predict the behavior of elements, their reactivity, and their properties. It serves as a valuable tool in chemistry research, education, and various applications in industry and technology.
Structure and organization of the periodic table
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is widely used in chemistry and is an essential tool for understanding the relationships between different elements and their properties.
The periodic table is structured in rows called periods and columns called groups. The periods are numbered from 1 to 7, and the elements in each period are organized in order of increasing atomic number. The groups are numbered from 1 to 18 and represent vertical columns of elements with similar properties.
The main organization of the periodic table is based on the electronic configuration of the elements. Each element is represented by its atomic symbol, atomic number, and atomic weight. The atomic symbol is a one or two-letter abbreviation for the element, while the atomic number represents the number of protons in the atom’s nucleus. The atomic weight is the average mass of the atom, taking into account the different isotopes of the element.
The periodic table also has several distinct regions or blocks:
- The s-block includes the first two groups (1 and 2) and the helium group (18). These elements have their outermost electrons in the s subshell.
- The p-block includes groups 13 to 18. These elements have their outermost electrons in the p subshell.
- The d-block includes groups 3 to 12 and is also called the transition metals block. These elements have their outermost electrons in the d subshell.
- The f-block is located below the main body of the periodic table and includes the lanthanides and actinides. These elements have their outermost electrons in the f subshell.
The periodic table provides a systematic way of organizing and studying the elements, making it easier to understand their properties and relationships. It allows scientists to predict and explain the behavior of elements and their compounds, leading to advancements in various fields such as materials science, medicine, and environmental studies.
How to read the periodic table?
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic numbers, electron configurations, and recurring chemical properties. By understanding the layout and symbols used in the periodic table, you can easily gather information about different elements and their characteristics.
- Element Symbol: Each element in the periodic table is represented by a symbol. These symbols can consist of one or two letters, often derived from the element’s name or its Latin name. For example, H represents hydrogen, O represents oxygen, and Fe represents iron.
- Atomic Number: The atomic number indicates the number of protons in the nucleus of the atom. It is usually located above or below the element symbol in the periodic table. The atomic number determines the element’s identity and its position in the table.
- Atomic Mass: The atomic mass represents the average mass of the element’s isotopes. It is usually located below the element symbol in the periodic table. The atomic mass is expressed in atomic mass units (amu).
- Periods: The periodic table is divided into periods, denoted by rows from 1 to 7. Each period corresponds to the energy level of the element’s outermost electrons. Elements in the same period tend to have similar electronic configurations.
- Groups/Families: The periodic table is also organized into groups or families, denoted by columns from 1 to 18. Elements in the same group share similar chemical properties due to their similar outer electron configurations.
Overall, the periodic table is a valuable tool for chemists and scientists to understand the properties and behaviors of various elements. It provides a systematic representation that allows for easy identification and comparison of different elements. By utilizing the information presented in the periodic table, scientists can predict the behavior of elements in chemical reactions and understand their roles in various natural and synthetic processes.
Understanding atomic number and atomic mass
The atomic number and atomic mass are two important properties of an element that can give us valuable information about its characteristics and behavior. Both of these values are found on the periodic table, which is a chart that organizes elements based on their atomic properties.
The atomic number represents the number of protons in an atom’s nucleus. Each element has a unique atomic number, which determines its place on the periodic table. Elements are ordered in ascending order of atomic number, from left to right and top to bottom. For example, hydrogen has an atomic number of 1, while helium has an atomic number of 2.
The atomic mass represents the total mass of an atom, including its protons, neutrons, and electrons. It is usually expressed in atomic mass units (amu). The atomic mass can vary for different isotopes of the same element, as isotopes have different numbers of neutrons. On the periodic table, the atomic mass is often shown as a decimal number below the element’s symbol. For example, the atomic mass of carbon is approximately 12.01 amu.
Understanding the atomic number and atomic mass is essential for various applications in chemistry and physics. The atomic number helps identify elements and their position in the periodic table, while the atomic mass is used for calculations involving chemical reactions, stoichiometry, and determining the number of atoms in a given sample. Additionally, isotopes with different atomic masses can have different properties and behaviors, making atomic mass an important factor in studying and understanding elements.
The significance of period and group numbers
In the periodic table, elements are organized based on their atomic number and electron configuration. The period number of an element indicates the highest energy level occupied by its electrons. Each period corresponds to a new electron shell being filled. The group number, on the other hand, indicates the number of valence electrons an element has. Valence electrons are the outermost electrons involved in chemical bonding.
Period numbers: The period number of an element provides information about its electron configuration and the number of occupied energy levels. The first period (Period 1) only contains two elements, hydrogen and helium, as they have only one and two electrons, respectively. Each subsequent period adds one more energy level until Period 7, which contains the elements with seven occupied energy levels.
Group numbers: The group number of an element can tell us about its electron configuration and chemical properties. Elements in the same group tend to have similar characteristics because they have the same number of valence electrons. For example, Group 1 elements (also known as alkali metals) all have one valence electron, which makes them highly reactive. Group 18 elements (also known as noble gases) have a full outer shell and are therefore chemically stable.
By understanding the significance of period and group numbers in the periodic table, scientists can predict certain properties and behaviors of elements based on their location and relationship to other elements. This information is crucial for studying and manipulating the behavior of elements in chemical reactions and for understanding the trends and patterns within the periodic table.
What are the elements in the periodic table?
The periodic table is a tabular arrangement of chemical elements organized based on their atomic number, electron configuration, and recurring chemical properties. It is a valuable tool for chemists and scientists to understand the relationships between different elements and their behavior.
The periodic table consists of various elements, each with its own unique set of properties. There are currently 118 known elements, out of which 94 occur naturally on Earth, and the rest have been synthesized in laboratories. These elements are classified into different categories based on their properties, such as metals, nonmetals, and metalloids.
Some of the most well-known elements include hydrogen, helium, oxygen, carbon, and nitrogen. These elements are essential for life as we know it and are commonly found in biological organisms. Other elements like gold, silver, and platinum are valuable for their rarity and unique physical properties. Elements like uranium and plutonium have radioactive properties and are used in nuclear reactors and weapons.
The periodic table also provides information about the atomic structure of elements, including their atomic mass, atomic radius, and electronegativity. It also highlights trends and patterns among elements, such as the periodicity of their properties and the periodic law, which states that the physical and chemical properties of elements are periodic functions of their atomic number.
In conclusion, the periodic table is a comprehensive and systematic arrangement of elements that allows scientists to study and understand the characteristics and behavior of different chemical substances. It is a fundamental tool in chemistry and provides a foundation for further scientific exploration and discovery.
Classification of elements into metals, non-metals, and metalloids
The periodic table of elements is a systematic arrangement of all the known chemical elements. One of the key aspects of the periodic table is the classification of elements into metals, non-metals, and metalloids.
Metals: Metals are characterized by their shiny appearance, high electrical and thermal conductivity, and malleability. They are typically found on the left side of the periodic table. Some common examples of metals include iron, copper, gold, and silver. Metals can also form positive ions by losing electrons.
Non-metals: Non-metals are generally poor conductors of heat and electricity. They are located on the right side of the periodic table. Non-metals exist in various forms, such as gases (oxygen, nitrogen), solids (sulfur, carbon), and liquids (bromine). Non-metals tend to gain electrons during a chemical reaction to achieve a stable electron configuration.
Metalloids: Metalloids, also known as semi-metals, have properties that are intermediate between those of metals and non-metals. They are found along the staircase-shaped line on the periodic table, separating the metals from the non-metals. Metalloids exhibit characteristics of both metals and non-metals, including semi-conductivity and variable conductivity depending on temperature and pressure.
In summary, the classification of elements into metals, non-metals, and metalloids is an important aspect of the periodic table. It helps in understanding and predicting the chemical properties and behaviors of different elements based on their grouping. This classification system provides a foundation for further exploration and study of the elements and their interactions in various chemical reactions.
Representative elements and transition elements
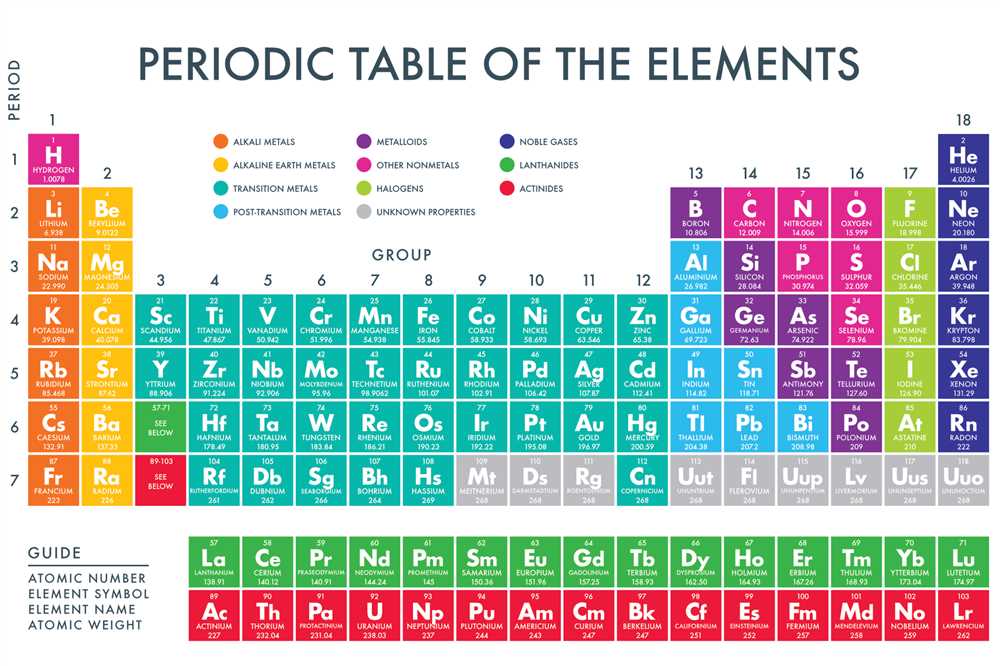
The periodic table is organized into several categories, including representative elements and transition elements. These categories are based on the electronic configurations of the elements and their properties.
Representative elements, also known as main group elements, are located in groups 1, 2, and 13 to 18 of the periodic table. They include elements such as hydrogen, lithium, carbon, oxygen, and chlorine. Representative elements have predictable properties and tend to exhibit similar chemical behavior within their respective groups. For example, group 1 elements, known as alkali metals, are highly reactive and tend to lose one electron to form a +1 cation.
Transition elements, also known as transition metals, are located in the middle of the periodic table, in groups 3 to 12. They include elements such as iron, copper, silver, and gold. Transition elements are characterized by their ability to form multiple oxidation states and their variable electronic configurations. They often exhibit colorful compounds and are known for their catalytic properties. Transition elements are essential for many biological processes and are used in various industrial applications.
Overall, the representative elements and transition elements play crucial roles in chemistry and offer a diverse range of properties and applications. The periodic table provides a systematic way of organizing these elements, allowing scientists to understand their behavior and predict their properties.