
The periodic table of elements is a fundamental tool in chemistry, organizing the building blocks of matter based on their atomic number, electron configuration, and chemical properties. Understanding the periodic table allows scientists to predict and explain the behavior of elements, as well as identify trends and patterns within the table.
Periodic trends refer to the trends or patterns that occur among the properties of elements as you move across a period or down a group in the periodic table. These trends provide valuable insights into the behavior of elements, including their reactivity, atomic size, and ionization energy.
This article explores several common questions related to periodic trends, providing answers and explanations to help deepen your understanding of this essential concept in chemistry. Whether you’re a student studying for an exam or a curious individual seeking to expand your knowledge, this collection of questions and answers in PDF format will be a valuable resource.
What are periodic trends?
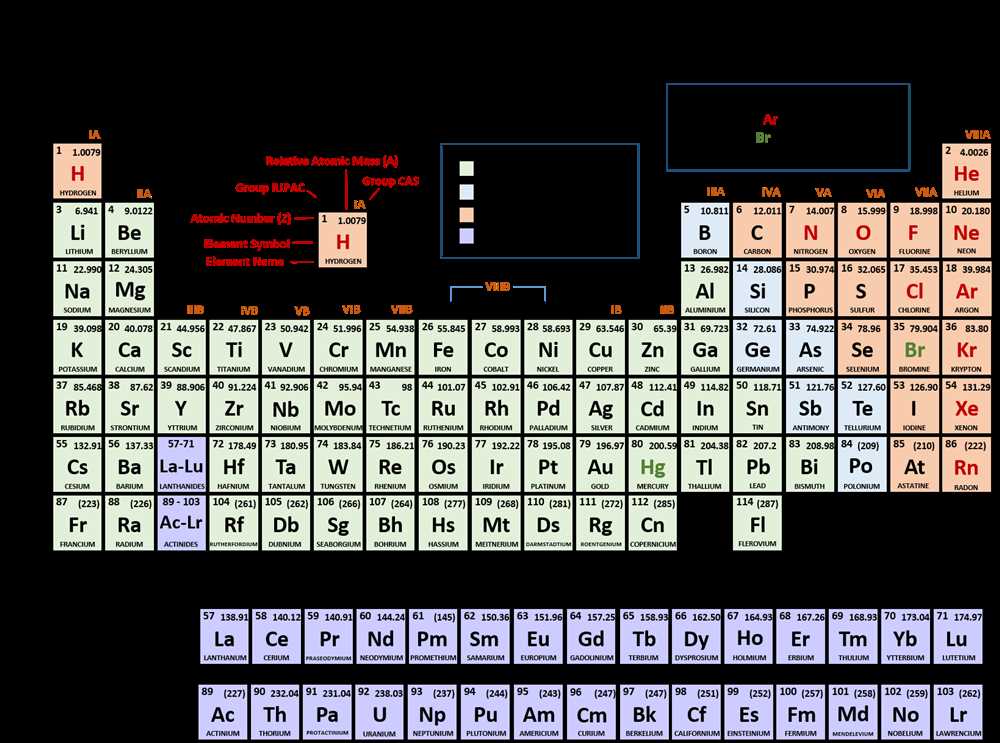
Periodic trends refer to the patterns that occur in the properties of elements as you move across or down the periodic table. These trends can be observed in various physical and chemical properties, such as atomic radius, ionization energy, electron affinity, and electronegativity. Understanding these trends is essential in predicting how elements will interact and react with each other.
One of the fundamental periodic trends is the atomic radius. As you move from left to right across a period, the atomic radius generally decreases. This is because the positively charged protons in the nucleus are attracting the negatively charged electrons more strongly, causing the electron cloud to be pulled in closer to the nucleus. Conversely, as you move down a group, the atomic radius increases. This is due to the addition of new energy levels as you go down the periodic table, resulting in a larger electron cloud.
Another important periodic trend is ionization energy, which is the energy required to remove an electron from an atom. Generally, ionization energy increases as you move from left to right across a period. This is because the electrons are held more tightly by the nucleus, making it more difficult to remove them. On the other hand, ionization energy decreases as you move down a group. This is because the outermost electrons are further from the nucleus and are shielded by inner electrons, making them easier to remove.
These are just a few examples of periodic trends that can be observed in the properties of elements. By studying and understanding these trends, chemists and scientists can make predictions about the behavior and reactivity of different elements. Periodic trends play a crucial role in various fields, such as materials science, pharmaceuticals, and environmental chemistry.
Importance of understanding periodic trends
Understanding periodic trends is crucial in the field of chemistry as it provides valuable insights into the behavior and properties of elements. These trends allow scientists to predict and explain various phenomena, which are essential for applications in fields such as material science, medicine, and environmental studies.
1. Predicting element properties: Periodic trends help in predicting the physical and chemical properties of elements. For example, understanding the periodic trend of atomic radius allows scientists to predict the size of atoms and ions, which affects their reactivity and ability to form chemical bonds. Similarly, knowledge of periodic trends in electronegativity helps in predicting the polarity of chemical bonds and the overall behavior of molecules.
2. Explaining periodic behavior: Periodic trends provide explanations for various trends and patterns observed in the periodic table. For instance, the periodic trend of ionization energy explains why elements in the same group have similar chemical reactivity. Elements with lower ionization energies are more likely to lose electrons and form positive ions, while elements with higher ionization energies tend to gain electrons and form negative ions.
3. Designing new materials: Understanding periodic trends is crucial for designing new materials with specific properties. By analyzing the periodic trends of elements, scientists can identify combinations of elements that are likely to exhibit certain properties, such as high conductivity or low reactivity. This knowledge allows for the development of new materials for applications ranging from electronics to medicine.
4. Environmental studies: Periodic trends also play a role in environmental studies, particularly in analyzing the behavior of elements in natural systems. For example, understanding the periodic trend of the melting and boiling points of elements helps in predicting their behavior in different environmental conditions. This knowledge is important for studying the impact of pollutants, as well as for understanding natural processes such as the movement of elements in the earth’s crust.
In conclusion, understanding periodic trends is of utmost importance in the field of chemistry. It enables scientists to predict element properties, explain periodic behavior, design new materials, and analyze environmental impact. Without this understanding, progress in various scientific and technological fields would be limited.
Why is it important to study periodic trends?
Studying periodic trends is crucial in the field of chemistry as it provides valuable insights into the behavior and properties of elements. These trends allow scientists to make predictions and understand the underlying principles behind various chemical phenomena.
1. Predicting element properties: Periodic trends, such as atomic radius, ionization energy, electronegativity, and electron affinity, provide information about how elements will interact with other substances. By studying these trends, scientists can predict the chemical reactivity, stability, and bonding capabilities of different elements.
2. Understanding periodic behavior: The periodic table organizes elements based on their electron configurations and atomic properties. By studying periodic trends, scientists can gain a deeper understanding of why certain elements behave in specific ways. For example, the periodic trend of increasing ionization energy across a period can explain why noble gases have low reactivity and are unlikely to form compounds.
3. Exploring chemical reactions: Periodic trends can provide insights into the likelihood and nature of chemical reactions. For instance, the trend of decreasing electronegativity down a group can explain the increasing reactivity of alkali metals as they readily lose electrons to form positive ions.
4. Designing materials and compounds: By studying periodic trends, scientists can design materials and compounds with specific properties. Understanding the periodic behavior of elements allows for the development of new materials with desirable characteristics, such as increased conductivity, enhanced strength, or improved stability.
5. Advancing scientific knowledge and applications: Further research into periodic trends can lead to the discovery of new elements, compounds, and phenomena. This knowledge can then be applied in various fields, including medicine, industry, and technology, to develop new materials, improve processes, and advance scientific understanding.
Overall, the study of periodic trends is essential for advancing our knowledge of chemistry and its applications. It provides a foundation for understanding the behavior and properties of elements and enables scientists to make predictions, design materials, and explore chemical reactions.
Periodic trend: Atomic radius
The atomic radius is a periodic trend referring to the size of an atom. It is defined as the distance between the nucleus and the outermost electron shell of an atom. The atomic radius generally decreases from left to right across a period and increases from top to bottom within a group on the periodic table.
As we move across a period, the atomic radius decreases. This is because the number of protons in the nucleus increases, attracting the electrons more strongly and pulling them closer to the nucleus. The increased nuclear charge offsets the shielding effect of inner electron shells, resulting in a smaller atomic radius.
- Key phrase: “atomic radius generally decreases from left to right across a period”
On the other hand, as we move down a group, the atomic radius increases. This is due to the addition of more electron shells as we move down the group. Each new electron shell increases the distance between the nucleus and the outermost electron, resulting in a larger atomic radius.
- Key phrase: “atomic radius increases from top to bottom within a group”
It is important to note that there may be exceptions to these trends due to specific electron configurations or other factors. However, in general, the periodic trend of atomic radius provides a useful framework for understanding the relative sizes of different atoms on the periodic table.
What is atomic radius?
An atomic radius refers to the size of an atom, specifically the distance between the nucleus and the outermost electron shell. It provides information about the size of an atom and can vary depending on the atom’s position in the periodic table.
The atomic radius generally increases as you move down a group on the periodic table. This is due to the addition of more electron shells, which increases the distance between the nucleus and the outermost electrons. As a result, the atoms become larger.
On the other hand, the atomic radius generally decreases as you move across a period on the periodic table from left to right. This is because the number of protons and electrons increases, leading to a stronger attractive force between the nucleus and the electrons. This contraction results in a smaller atomic radius.
The atomic radius is an important concept in chemistry as it affects various physical and chemical properties of atoms, such as ionization energy, metallic character, and chemical reactivity. Understanding the trends in atomic radius can provide insights into the behavior of elements and their compounds.
Periodic trend: Ionization energy
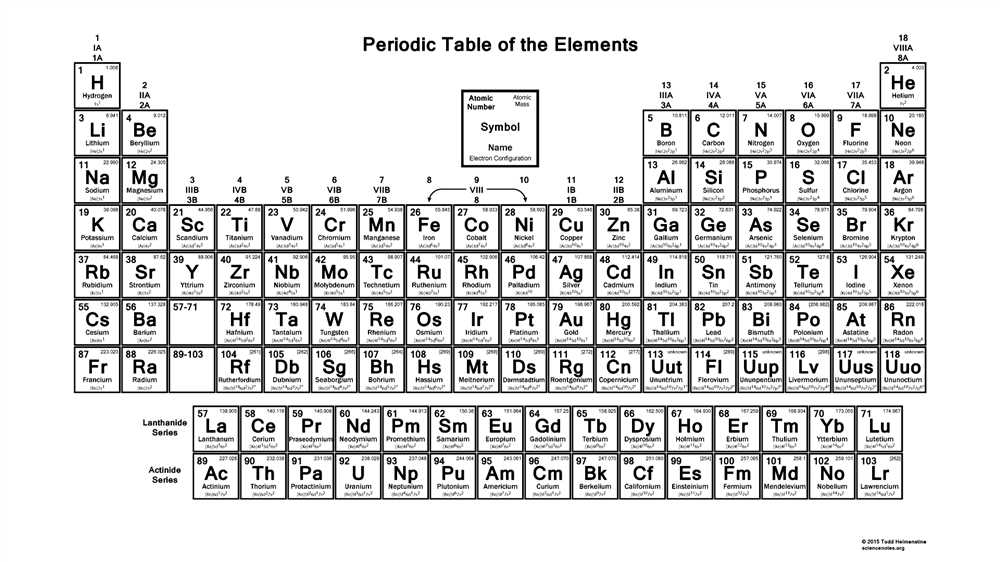
The ionization energy is the energy required to remove an electron from a neutral atom, resulting in the formation of a positive ion. This property is an important factor in understanding the periodic behavior of elements.
The general trend: Ionization energy generally increases across a period from left to right and decreases down a group in the periodic table. This trend is primarily influenced by the atomic radius and the effective nuclear charge experienced by the outermost electrons.
- Across a period: As we move from left to right across a period, the atomic radius decreases, resulting in stronger nuclear attraction on the outermost electrons. It requires more energy to remove an electron from a smaller atom, leading to increasing ionization energy.
- Down a group: Going down a group, the atomic radius increases due to the addition of more energy levels. The outermost electrons are further away from the positively charged nucleus, resulting in weaker nuclear attraction. As a result, it becomes easier to remove an electron, and the ionization energy decreases.
There are, however, exceptions to this general trend due to certain factors such as electron configuration stability and electron repulsion. For example, elements with half-filled or completely filled subshells have lower ionization energies than expected due to increased stability.
| Period | Trend in ionization energy |
|---|---|
| Period 2 (Li to Ne) | Generally increases |
| Period 3 (Na to Ar) | Generally increases |
| Period 4 (K to Kr) | Generally increases |
| Period 5 (Rb to Xe) | Generally increases |
| Period 6 (Cs to Rn) | Generally increases |
In summary, ionization energy is an important periodic trend that reflects the energy required to remove an electron from an atom. It generally increases across a period and decreases down a group in the periodic table. However, there are exceptions to this trend due to factors such as electron configuration stability. Understanding these trends helps to explain the behavior and properties of elements.
What is ionization energy?
Ionization energy is the amount of energy required to remove an electron from an atom or ion in the gaseous state. It is a measure of the strength of the attraction between the negatively charged electron and the positively charged nucleus. The ionization energy is dependent on the atomic radius, electron configuration, and nuclear charge of an atom or ion.
The ionization energy generally increases across a period and decreases down a group in the periodic table. This is due to the trend in atomic size and effective nuclear charge. As the atomic radius decreases across a period, the electron is held more tightly by the nucleus, resulting in higher ionization energy. Similarly, as you move down a group, the atomic radius increases and the electron is held less tightly by the nucleus, leading to lower ionization energy.
The ionization energy values can be used to predict the reactivity and chemical properties of elements. Elements with low ionization energies are more likely to lose electrons and form positive ions, while elements with high ionization energies are less likely to lose electrons and form negative ions. Additionally, the ionization energy can be used to identify the position of an element in the periodic table and compare its reactivity to other elements.
Overall, ionization energy is an important concept in understanding the behavior of elements and their chemical reactions. It provides insight into the stability and reactivity of atoms and ions, and helps in predicting the properties and behavior of different elements in various chemical processes.
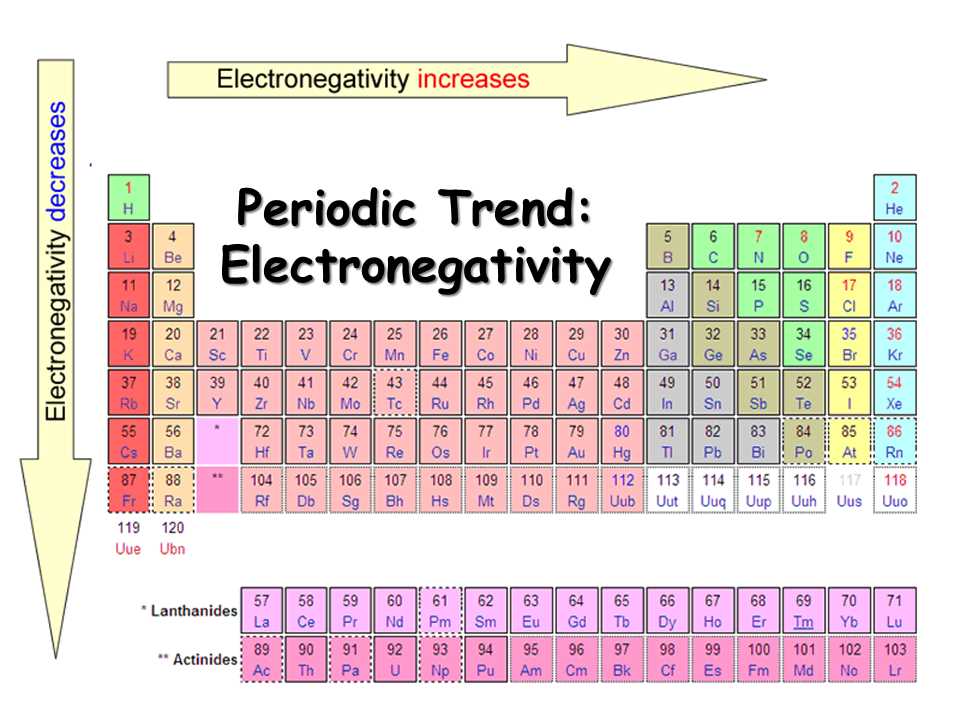
Periodic trend: Electronegativity
Electronegativity refers to the ability of an atom to attract shared electrons in a chemical bond. It is an important concept in chemistry as it helps us understand the behavior of elements and their chemical reactivity. Electronegativity values are assigned to each element on the periodic table, reflecting their relative ability to attract electrons.
The electronegativity of an element follows a periodic trend, meaning that it tends to increase or decrease as one moves across or down the periodic table, respectively. This trend is influenced by factors such as atomic size, nuclear charge, and electron shielding. Generally, electronegativity tends to increase from left to right across a period and decrease from top to bottom within a group.
Knowing the electronegativity trend can help predict the types of chemical bonds that will form between elements. In general, when elements with large differences in electronegativity bond together, they form ionic bonds, where one element gains electrons and the other loses electrons. On the other hand, when elements with similar electronegativity values bond, they form covalent bonds, where electrons are shared between atoms.
Electronegativity values can also be used to predict the polarity of bonds and molecules. A bond or molecule is considered polar if there is an unequal distribution of electron density, leading to a partial positive and partial negative charge. This occurs when there is a significant difference in electronegativity between the atoms involved in the bond or molecule.
In summary, electronegativity is a periodic trend that indicates an atom’s ability to attract electrons. Understanding this trend is crucial for studying chemical bonding, predicting the types of bonds that will form, and determining the polarity of molecules.