
Welcome to this comprehensive study guide for your Pharmacology Exam 2. This guide has been designed to help you review and consolidate your knowledge in pharmacology, allowing you to feel confident and prepared for the upcoming exam. Whether you are a medical student, a pharmacy student, or studying any other healthcare-related field, this guide will provide you with the essential information needed to succeed on your exam.
This study guide covers a wide range of topics in pharmacology, including drug classifications, mechanisms of action, pharmacokinetics, and therapeutic uses. Each topic is broken down into manageable sections, with key points and summaries provided to help you quickly grasp and retain the material. Additionally, the guide includes practice questions and examples to test your understanding and apply the concepts you have learned.
By using this study guide, you will be able to effectively review the material covered in the course and identify any areas that you may need to focus on. It is designed to be a supplement to your lectures and textbooks, providing you with a concise and organized overview of the most important information. Whether you are studying alone or in a group, this guide can serve as a valuable tool to optimize your study time and ensure success on your Pharmacology Exam 2.
Overview of Pharmacology Exam 2
In the second pharmacology exam, students will be tested on their understanding of various concepts related to the field of pharmacology. This exam will focus on topics such as drug absorption, distribution, metabolism, and excretion, as well as drug interactions and adverse effects. Students will also be expected to demonstrate their knowledge of the different classes of drugs, including their mechanisms of action and therapeutic uses.
One key area of focus for this exam is pharmacokinetics, which refers to the study of how drugs are absorbed, distributed, metabolized, and excreted by the body. Students will be expected to understand the different factors that can affect drug absorption, such as route of administration, drug solubility, and drug interactions. They will also need to be familiar with the various ways in which drugs are distributed throughout the body, including the role of plasma protein binding and tissue distribution.
Another important topic covered in this exam is pharmacodynamics, which examines how drugs interact with specific targets in the body to produce their effects. Students will need to understand the concept of drug-receptor interactions, as well as the different types of receptors and signaling pathways involved. They will also be expected to demonstrate their knowledge of the mechanisms of action of different drug classes, including agonists, antagonists, and enzyme inhibitors.
In addition to pharmacokinetics and pharmacodynamics, this exam will also assess students’ understanding of drug interactions, including drug-drug interactions, drug-food interactions, and drug-herb interactions. Students will need to be able to identify potential drug interactions and recommend appropriate management strategies in order to minimize the risk of adverse effects.
Overall, the second pharmacology exam will test students’ knowledge and understanding of key concepts and principles in pharmacology. It is important for students to review and study the relevant material in order to successfully pass this exam.
Basic Concepts in Pharmacokinetics
Pharmacokinetics is the study of how drugs are absorbed, distributed, metabolized, and excreted in the body. Understanding these processes is essential in determining the appropriate dosage and dosing intervals for a drug. There are several key concepts in pharmacokinetics that are important to grasp in order to effectively administer medications.
Firstly, absorption refers to the process by which drugs are taken up into the bloodstream. The rate and extent of absorption can vary depending on factors such as the drug’s physical and chemical properties, the route of administration, and the individual’s physiological characteristics. For example, drugs administered intravenously are immediately available in the bloodstream, while drugs taken orally may be subject to first-pass metabolism in the liver before reaching the systemic circulation.
Distribution refers to how drugs are transported throughout the body. Factors that influence drug distribution include the drug’s ability to penetrate various tissues and organs, as well as the presence of protein binding and drug interactions. Drug distribution is primarily determined by blood flow, so tissues with high blood flow receive more drug than those with lower blood flow. Understanding a drug’s distribution profile is crucial in assessing its potential therapeutic effects and side effects.
Metabolism, also known as biotransformation, involves the enzymatic breakdown of drugs into metabolites that can be more easily eliminated from the body. The liver is the primary site of drug metabolism, although other organs such as the kidneys, intestines, and lungs can also play a role. Factors that influence drug metabolism include genetics, age, gender, and the presence of other drugs. Understanding a drug’s metabolic pathway is important in assessing its potential for drug-drug interactions and in predicting its pharmacological activity.
Excretion is the final step in drug elimination from the body. The kidneys are the main organs responsible for drug excretion through urine, although drugs can also be eliminated through feces, sweat, breast milk, and exhaled air. The rate of drug excretion is primarily determined by factors such as renal blood flow, glomerular filtration rate, and tubular secretion. Assessing a drug’s excretion profile can help determine appropriate dosing intervals and adjust drug therapy in patients with renal impairment.
Overall, a thorough understanding of pharmacokinetic principles is essential for healthcare professionals to ensure safe and effective drug therapy. By considering factors such as absorption, distribution, metabolism, and excretion, clinicians can optimize drug dosing regimens and minimize the risk of adverse effects.
Absorption
Absorption is the process by which a drug enters the bloodstream and becomes available for distribution to the target tissues. It is the first stage in the pharmacokinetic process, following administration of a drug. Absorption can occur through various routes, including oral, topical, intravenous, subcutaneous, and inhalation.
Oral absorption is the most common route of administration for drugs, where the drug is taken by mouth and passes through the gastrointestinal tract. The rate and extent of absorption can be influenced by several factors, such as the solubility and stability of the drug, the presence of food in the stomach, and the pH of the gastrointestinal tract.
Topical absorption occurs when a drug is applied to the skin or mucous membranes. This route is commonly used for local effects, but some drugs can also be absorbed systemically. The rate and extent of absorption through the skin can be influenced by factors such as the integrity of the skin barrier, the concentration of the drug, and the vehicle used for drug delivery.
Intravenous absorption is the fastest and most direct route of administration, where the drug is injected into the bloodstream. This route allows for rapid and complete absorption of the drug, as it bypasses the barriers of the gastrointestinal tract and skin.
Subcutaneous absorption occurs when a drug is injected into the layer of tissue beneath the skin. This route allows for slower and sustained absorption of the drug, as it is released into the systemic circulation over time.
Inhalation absorption occurs when a drug is inhaled into the lungs. This route is commonly used for respiratory medications, as it allows for direct delivery of the drug to the target tissues in the respiratory system. The rate and extent of absorption through inhalation can be influenced by factors such as the particle size of the drug, the depth of inhalation, and the presence of lung diseases.
Overall, the absorption of a drug is an important factor to consider in pharmacology, as it determines the bioavailability and onset of action of the drug. Understanding the different routes of absorption and factors that can influence absorption can help optimize drug therapy and patient outcomes.
Distribution
After absorption, drugs are distributed throughout the body via the bloodstream. The distribution of a drug is influenced by factors such as its molecular size, solubility, and binding affinity to proteins. Some drugs have a high affinity for binding to plasma proteins such as albumin, which can lead to decreased availability of the free, unbound form of the drug.
Drugs can also be distributed into various body compartments such as the central nervous system (CNS), which is protected by the blood-brain barrier. The blood-brain barrier restricts the passage of many drugs, but certain drugs with specific characteristics, such as low molecular weight and lipid solubility, are able to cross this barrier and reach therapeutic concentrations in the CNS.
Some drugs may undergo further distribution into specific organs or tissues, such as the liver or adipose tissue, where they may accumulate. This can have implications for drug efficacy and toxicity, as drugs that accumulate in certain tissues may have prolonged effects or increased risk of adverse reactions.
Understanding the distribution of drugs is important for determining their therapeutic efficacy, as well as their potential for side effects and interactions with other drugs. Pharmacokinetic studies help to characterize the distribution patterns of drugs and provide valuable information for dose adjustment and individualized treatment. In addition, factors such as patient characteristics and disease states can also influence drug distribution, highlighting the importance of personalized medicine in optimizing drug therapy.
Metabolism
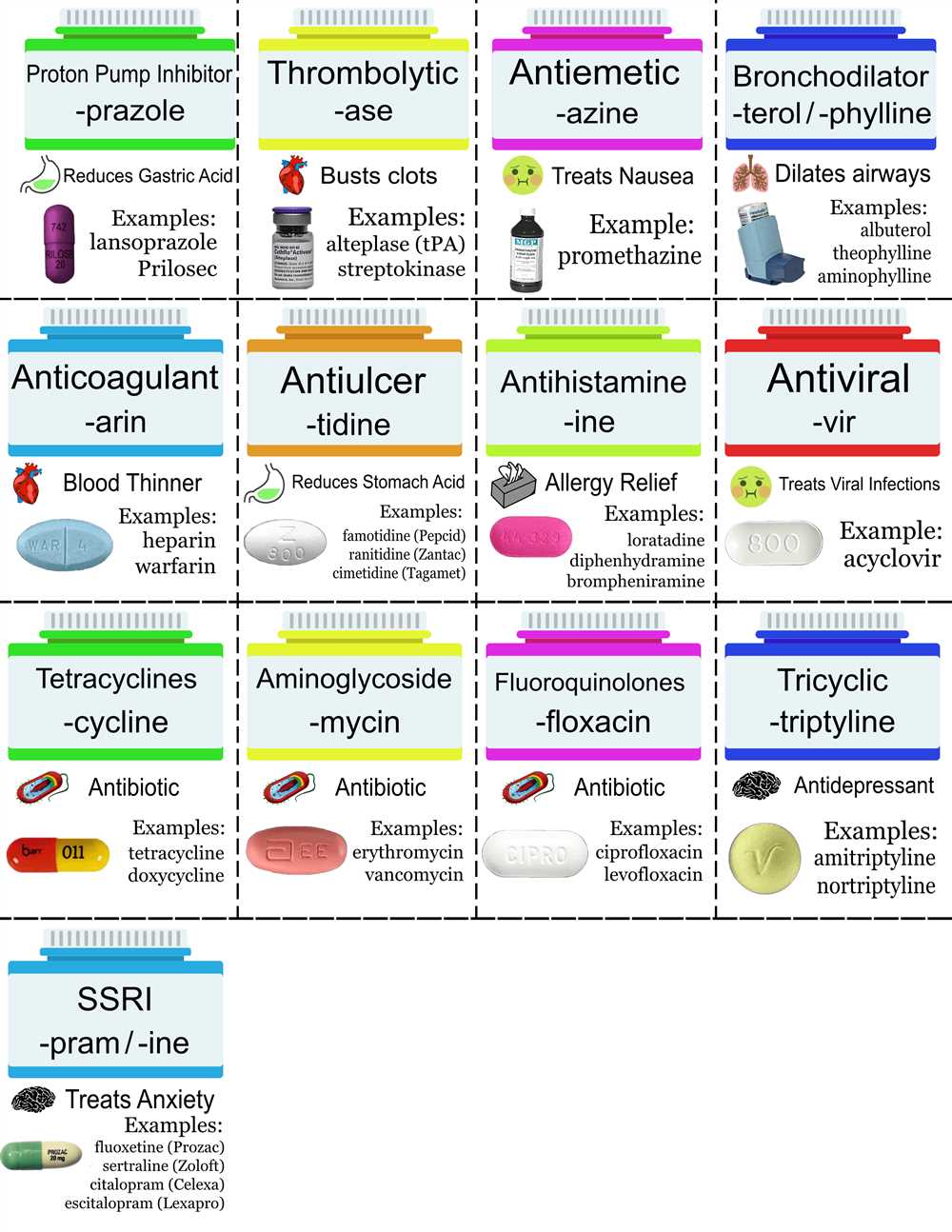
Metabolism is the process by which the body breaks down chemicals and converts them into energy or other useful substances. It is a complex series of chemical reactions that occur in all living organisms, including humans. Understanding metabolism is essential in pharmacology because it helps us understand how drugs are broken down and eliminated from the body.
The liver is the primary organ responsible for metabolism in the body. It contains enzymes that facilitate the breakdown of drugs, as well as other foreign substances, into their metabolites. These metabolites are usually less active or inactive compared to the parent drug. Metabolism can result in the activation of drugs as well, where they are converted into their active form.
There are two main types of metabolism: Phase I and Phase II. Phase I metabolism involves oxidation, reduction, or hydrolysis reactions. These reactions often introduce a functional group to the drug molecule, making it more water-soluble and easier to excrete. Phase II metabolism involves conjugation reactions, where the drug or its metabolite combines with an endogenous substance, such as glucuronic acid or sulfur, to form a conjugate. These conjugates are highly water-soluble and easily eliminated from the body.
Metabolism can be influenced by various factors, such as age, genetics, disease states, and drug interactions. For example, some individuals may have genetic variations that result in decreased metabolism of certain drugs, leading to higher drug concentrations and increased risk of toxicity. Drug interactions can also affect metabolism, either by inhibiting or inducing the activity of drug-metabolizing enzymes.
Summary:
- Metabolism is the process by which the body breaks down chemicals and converts them into energy or other useful substances.
- The liver is the primary organ responsible for metabolism in the body.
- There are two main types of metabolism: Phase I and Phase II.
- Factors such as age, genetics, disease states, and drug interactions can influence metabolism.
Elimination
Elimination is the process by which a drug or its metabolites are removed from the body. It is an important aspect of pharmacokinetics as it determines the duration and intensity of drug action. There are different routes of elimination, including renal excretion, hepatic metabolism, and biliary excretion.
Renal excretion is the most common route of elimination for drugs. The kidneys filter the drug and its metabolites from the bloodstream into the urine, which is then excreted from the body. This process depends on many factors, such as the drug’s molecular size, charge, and lipid solubility. Some drugs are actively secreted from the blood into the urine by renal tubular cells, increasing their elimination.
Hepatic metabolism is another important route of elimination. The liver plays a crucial role in metabolizing drugs into less active or inactive forms, which are then eliminated from the body. This process involves the cytochrome P450 enzymes, which catalyze the biotransformation of drugs. Some drugs undergo extensive metabolism in the liver, while others may undergo little or no metabolism.
Biliary excretion is the process by which drugs and their metabolites are excreted into the bile, which is then eliminated from the body through feces. This route of elimination is especially important for drugs that are highly lipophilic or not efficiently eliminated by renal excretion. The drug or its metabolites may be reabsorbed from the intestines back into the bloodstream, a process known as enterohepatic recycling.
In summary, elimination is the process by which drugs are removed from the body. It involves renal excretion, hepatic metabolism, and biliary excretion. Understanding the routes of elimination is essential for predicting drug clearance and optimizing drug therapy.
Drug-Receptor Interactions

The interaction between drugs and receptors is a fundamental concept in pharmacology. A receptor, typically located on the surface or inside a cell, is a protein molecule that can recognize and bind to specific drugs or endogenous ligands. This binding triggers a series of events that ultimately lead to a biological response. Understanding the drug-receptor interaction is crucial for predicting the pharmacological effects of a drug and optimizing its therapeutic use.
There are different types of drug-receptor interactions, including agonist, antagonist, and inverse agonist interactions. An agonist is a drug that binds to a receptor and activates it, producing a physiological response. On the other hand, an antagonist is a drug that binds to a receptor but does not activate it, preventing the binding of other ligands and blocking the physiological response. An inverse agonist, in contrast, binds to a receptor and produces the opposite effect of an agonist, reducing basal activity.
- Agonist interactions: Agonist drugs bind to receptors and activate them, mimicking the effect of endogenous ligands. This leads to a biological response and often results in therapeutic effects. Examples of agonist drugs include opioids, which bind to opioid receptors and relieve pain, and beta-blockers, which bind to beta-adrenergic receptors and reduce heart rate and blood pressure.
- Antagonist interactions: Antagonist drugs bind to receptors but do not activate them. Instead, they block the binding of other ligands, preventing the physiological response. Antagonists can be competitive, meaning they compete with endogenous ligands for receptor binding sites, or they can be non-competitive, meaning they bind to a different site on the receptor and inhibit its function. An example of a competitive antagonist is naloxone, which reverses the effects of opioids by binding to opioid receptors and displacing the opioid drug. An example of a non-competitive antagonist is phenoxybenzamine, which irreversibly binds to alpha-adrenergic receptors and inhibits their function.
- Inverse agonist interactions: Inverse agonists bind to receptors and produce the opposite effect of an agonist. They reduce the basal activity of the receptor, which can be beneficial in certain conditions where there is excessive receptor activity. For example, inverse agonists of the histamine H2 receptor are used to reduce gastric acid secretion in the treatment of peptic ulcers.