In the study of chemistry, understanding molecular shapes is crucial in predicting the behavior and properties of substances. Phet molecule shapes worksheet is a widely used tool to help students practice and master the concept of molecular shapes. This worksheet focuses on providing students with a hands-on approach to explore various molecules and their shapes using the Phet interactive simulation.
The answer key for the Phet molecule shapes worksheet in pdf format is a valuable resource for both students and teachers. It provides a comprehensive guide to help students check their answers and understand the reasoning behind each answer. This answer key covers all the questions and exercises presented in the worksheet, ensuring that students can fully grasp the concept of molecular shapes.
The Phet molecule shapes worksheet answer key pdf is an excellent tool for self-study and review. Students can use it to practice their understanding and identify areas of weakness before quizzes or exams. Teachers can also utilize it to assess students’ comprehension and provide additional guidance in areas where clarification is needed. The concise and clear format of the answer key makes it easy to navigate and understand.
Phet Molecule Shapes Worksheet Answer Key PDF

Phet Molecule Shapes Worksheet is a popular resource used in chemistry education to help students understand the three-dimensional shapes of molecules. This worksheet provides practice problems and activities for students to explore different molecular geometries and bond angles. To check their answers and gauge their understanding, an answer key in PDF format is provided.
The answer key in PDF format allows students to easily access and review the correct answers to the worksheet questions. It provides a comprehensive guide that helps students understand the concept of molecular shapes and how to determine them using different methods such as Lewis structures and VSEPR theory. The PDF format also allows for easy printing and sharing of the answer key, making it a convenient resource for both teachers and students.
With the Phet Molecule Shapes Worksheet Answer Key in PDF format, students can effectively practice and reinforce their understanding of molecular shapes. They can check their answers and identify any misconceptions they may have, allowing them to correct their mistakes and improve their understanding. The answer key also serves as a valuable tool for teachers, who can use it to assess their students’ progress and provide targeted feedback.
Understanding the Phet Simulation
The Phet molecule shapes simulation is a powerful tool for understanding the three-dimensional structures of molecules. By manipulating different atoms and bonds, students can explore how different arrangements lead to different shapes and properties. This interactive simulation allows students to visualize and manipulate molecules in real-time, providing them with a hands-on experience that enhances their understanding of chemical concepts.
Key Features:
- Atom selection: The simulation allows students to choose from a variety of atoms, including hydrogen, oxygen, carbon, and more. This feature enables students to create molecules with different compositions and explore how the shape changes accordingly.
- Bond formation: Students can connect atoms to form bonds, simulating the process of chemical bonding. This feature helps students understand the relationship between bond type and molecular shape.
- Molecular visualization: The simulation provides a 3D representation of the molecules, allowing students to view them from different angles. This feature helps students develop spatial reasoning skills and gain a deeper understanding of molecular structures.
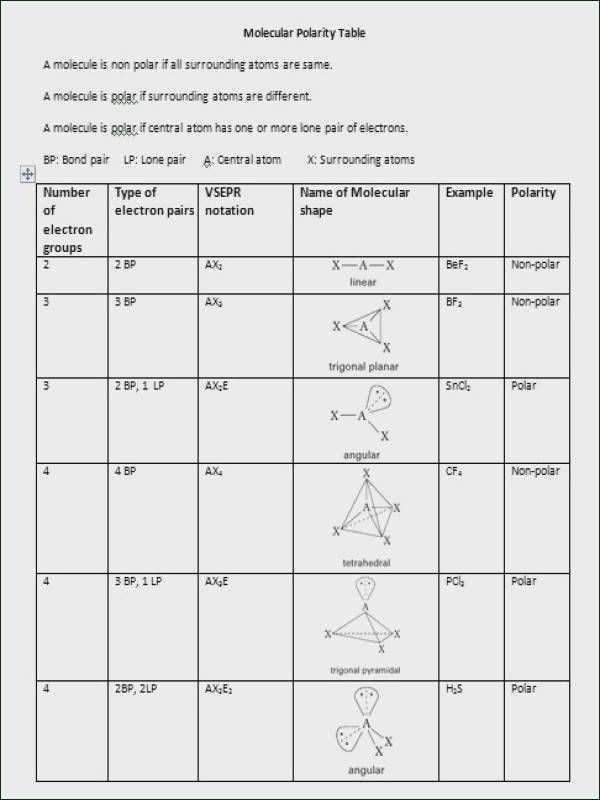
- Properties exploration: Students can explore the properties of different molecules, such as polarity and symmetry. This feature allows students to make connections between molecular shape and physical and chemical properties.
Overall, the Phet molecule shapes simulation is a valuable tool for teaching and learning about molecular structures. It engages students in an interactive and visual way, promoting a deeper understanding of chemistry concepts. By actively manipulating atoms and visualizing the resulting molecules, students can explore the relationships between structure and function, and deepen their understanding of the molecular world.
The Importance of Molecular Geometry

Molecular geometry plays a crucial role in understanding the behavior and properties of molecules. It refers to the three-dimensional arrangement of atoms within a molecule, which determines its overall shape and how it interacts with other molecules. By analyzing the molecular geometry, scientists can gain valuable insights into a molecule’s physical and chemical properties, such as its polarity, reactivity, and ability to form bonds.
Molecular geometry influences a molecule’s polarity. Polarity refers to the separation of electric charges within a molecule, which affects its interactions with other molecules. Molecules with polar bonds can be either polar or nonpolar, depending on their overall shape. For example, a molecule with a linear shape and symmetric distribution of charges, like carbon dioxide (CO2), is nonpolar, whereas a molecule with a bent shape and an uneven distribution of charges, like water (H2O), is polar. Understanding the molecular geometry is crucial for predicting a molecule’s polarity, which in turn influences its solubility, boiling point, and chemical reactivity.
Molecular geometry determines a molecule’s reactivity and bonding behavior. The arrangement of atoms within a molecule affects its ability to form and break chemical bonds. Different molecular geometries result in different bond angles and distances, which determine the strength and stability of the bonds. For example, molecules with tetrahedral geometry, like methane (CH4), have bond angles of 109.5 degrees and form strong covalent bonds. In contrast, molecules with linear geometry, like carbon monoxide (CO), have bond angles of 180 degrees and form weaker triple bonds. Understanding the molecular geometry allows scientists to predict how a molecule will interact with other molecules, whether it will form stable bonds or react with certain compounds.
- Shape determines the physical properties of molecules. The overall shape of a molecule affects its boiling point, melting point, and surface tension. For example, linear molecules tend to have higher boiling points and melting points compared to branched molecules, as the linear arrangement allows for greater surface contact and stronger intermolecular forces. Additionally, molecular geometry influences the surface tension of a substance, which determines its ability to spread or bead up on a surface. By understanding the molecular geometry, scientists can predict and manipulate these physical properties for various applications, such as designing materials with specific melting points or creating surfactants with specific surface tension characteristics.
In summary, molecular geometry is of utmost importance in understanding the properties and behavior of molecules. It provides valuable information about a molecule’s polarity, reactivity, bonding behavior, and physical properties. By studying and manipulating molecular geometry, scientists can make significant advancements in various fields, including chemistry, materials science, and drug discovery.
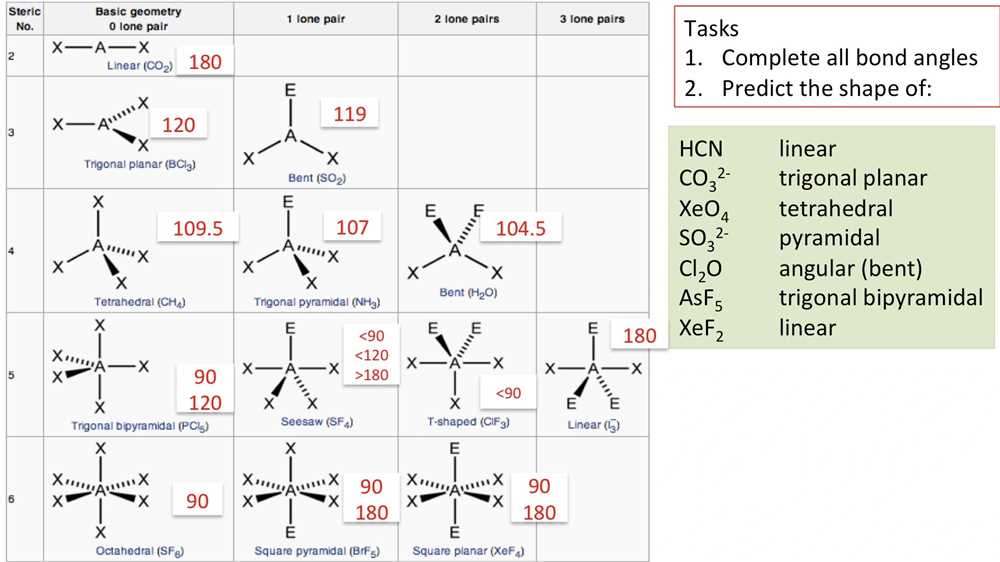
Key concepts in the Molecule Shapes worksheet help students understand the shapes of different molecules and the factors that determine these shapes. By understanding these concepts, students can predict the molecular geometry and bond angles of molecules, which is important in understanding their physical and chemical properties.
One key concept in the worksheet is the idea that the shape of a molecule is determined by the repulsion between electron pairs. This concept is based on the VSEPR theory, which stands for “valence shell electron pair repulsion.” According to this theory, electron pairs around a central atom repel each other and arrange themselves as far apart as possible, resulting in specific molecular shapes.
The worksheet also introduces students to different types of electron pairs, including bonding pairs and lone pairs. Bonding pairs are the shared electrons that form a bond between two atoms, while lone pairs are non-bonding electrons that reside on the central atom. The presence of lone pairs can affect the molecule’s shape and bond angles.
Using the worksheet, students can practice identifying the number of electron pairs and lone pairs around a central atom, and then use this information to determine the molecular shape. Some of the common molecular shapes covered in the worksheet include linear, trigonal planar, tetrahedral, trigonal pyramidal, and bent shapes.
Step-by-Step Instructions for the Worksheet
This worksheet is designed to help students understand and practice the concepts of molecule shapes using the Phet interactive simulation. By following these step-by-step instructions, students will be able to explore and determine the shape of various molecules, understand the factors that influence molecular shape, and apply their knowledge to real-world examples.
Step 1: Access the Phet Simulation
To begin, students should access the Phet website and open the “Molecule Shapes” simulation. This interactive tool allows students to build molecules and visualize their shapes in 3D.
Step 2: Familiarize with the Simulation
Before starting the worksheet, it’s essential for students to spend some time familiarizing themselves with the simulation’s interface and features. They should explore the different tools and options available, such as adding atoms, adjusting bond angles, and rotating the molecule.
Step 3: Complete the Worksheet Questions
The worksheet consists of a series of questions that guide students through the process of determining the shape of specific molecules. Students should carefully read and analyze each question, use the simulation to build the molecule, and then record their findings. They should pay close attention to the number of bonding pairs and lone pairs of electrons surrounding the central atom, as these factors determine the molecular shape.
Step 4: Check the Answer Key
After completing the worksheet, students can check their answers using the provided answer key. This will allow them to evaluate their understanding and identify any areas that may require further clarification or practice.
Step 5: Reflect and Apply
Finally, students should take some time to reflect on their learning and apply their knowledge to real-world examples. They can discuss and analyze the shapes of molecules found in everyday substances, such as water, carbon dioxide, and ammonia. This will help solidify their understanding of molecular shape and its importance in various chemical reactions and properties.
Not understanding the instructions and rushing through the worksheet can lead to common mistakes when completing the Phet molecule shapes worksheet. It is important to carefully read the instructions and understand the concepts before starting. Take your time to analyze the molecule shapes and their corresponding names.
One common mistake is not correctly identifying the shape of the molecule. This can occur if you do not pay attention to the arrangement of the atoms and the number of bonding and non-bonding electron pairs. It is important to carefully count the electron pairs and verify the shape using the provided key or reference materials.
Another mistake to avoid is not properly filling in the worksheet. Make sure to write down the correct molecular formula and number of valence electrons associated with each molecule. Additionally, double-check your answers to ensure you have matched the correct shape to each molecule.
Inaccurate labeling of the molecular geometry can also be a common mistake. Take the time to correctly identify the positions of the atoms and their bond angles. Pay attention to the positions of lone pairs and how they affect the overall shape of the molecule.
Lastly, rushing through the worksheet and not double-checking your answers can lead to careless mistakes. It is crucial to review your work and verify that you have completed the worksheet accurately. Take the time to go back and review any confusing or challenging concepts.
By being mindful of these common mistakes and taking the necessary steps to avoid them, you can ensure a more successful completion of the Phet molecule shapes worksheet. Double-checking your work, understanding the concepts, and paying attention to the details are key to achieving accurate results.
Answer Key for Phet Molecule Shapes Worksheet

The Phet Molecule Shapes Worksheet is a hands-on activity that allows students to explore the shapes of molecules and understand how these shapes affect their properties and behavior. The accompanying answer key provides the correct solutions to the questions and exercises in the worksheet, helping students to check their understanding and assess their progress.
Here are some examples of the answer key for the Phet Molecule Shapes Worksheet:
- Question: What is the electron domain geometry and molecular geometry of a molecule with two bonding domains and no lone pairs?
Answer: The electron domain geometry is linear, while the molecular geometry is also linear.
- Question: How does the presence of lone pairs affect the molecular geometry of a molecule with three bonding domains?
Answer: The presence of lone pairs can distort the molecular geometry, causing it to deviate from the perfect trigonal planar shape. For example, if there is one lone pair, the molecular geometry can become bent or angular, while if there are two lone pairs, the molecular geometry can become trigonal pyramidal.
- Question: What is the electron domain geometry and molecular geometry of a molecule with four bonding domains and one lone pair?
Answer: The electron domain geometry is tetrahedral, while the molecular geometry is trigonal pyramidal.
This answer key serves as a valuable resource for both students and instructors, allowing for a better understanding of the concepts covered in the Phet Molecule Shapes Worksheet and aiding in the assessment of student knowledge and progress in the study of molecular shapes.