
In this lab, we conducted a series of experiments to investigate physical and chemical changes. We were able to observe and document the various changes that occurred during these experiments, and this article will provide a detailed analysis of our findings.
One of the experiments involved heating different substances and observing their reactions. We observed that some substances underwent physical changes, such as melting or boiling, while others underwent chemical changes, such as the emission of gas or the formation of a new substance. Through careful observation and documentation, we were able to determine the specific changes that occurred in each substance.
Another experiment involved mixing different substances together and noting any changes that occurred. We observed that some mixtures resulted in physical changes, such as changes in color or texture, while others resulted in chemical changes, such as the formation of a precipitate or the release of heat. By analyzing the results of these mixtures, we were able to identify the specific changes that took place.
In conclusion, this lab provided us with a deeper understanding of physical and chemical changes. Through our experiments and observations, we were able to distinguish between different types of changes and identify their characteristics. This knowledge will be valuable in further scientific studies and applications.
Physical and Chemical Changes Lab Answers
During a physical and chemical changes lab, various substances and reactions are observed to determine whether they result in a physical change or a chemical change. In a physical change, the substance remains the same chemically but undergoes a change in its physical properties, such as shape, size, or state of matter. On the other hand, a chemical change involves a rearrangement of atoms or molecules, leading to the formation of new substances with different chemical properties.
One of the experiments conducted in the lab involved heating a piece of magnesium metal in a Bunsen burner flame. The initial substance, magnesium metal (Mg), underwent a chemical change as it reacted with oxygen in the air to form magnesium oxide (MgO). This reaction is represented by the following chemical equation: 2Mg + O2 → 2MgO. The formation of a new substance with different chemical properties clearly indicates a chemical change.
In another experiment, a small piece of wax was melted in a test tube placed in a boiling water bath. The wax underwent a physical change as its state of matter changed from solid to liquid upon heating. The chemical composition of the wax remained the same, only the physical properties, such as its shape and state, were altered. Therefore, this experiment demonstrated a physical change.
The lab also involved the reaction between vinegar (acetic acid) and baking soda (sodium bicarbonate). As the two substances came into contact, bubbles of carbon dioxide gas were produced, indicating a chemical change. The reaction can be represented by the following equation: CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O. The formation of a new substance (sodium acetate) and the production of gas clearly demonstrate a chemical change.
In conclusion, physical and chemical changes can be distinguished based on the presence of new substances with different chemical properties and the rearrangement of atoms or molecules. The observations made during a physical and chemical changes lab help us understand how various substances behave under different conditions and the types of changes that occur.
Definition of Physical and Chemical Changes in Chemistry
In chemistry, physical and chemical changes are two types of transformations that matter can undergo. These changes involve alterations in the physical and chemical properties of substances, resulting in the formation of new products. While physical changes only affect the physical properties of matter, chemical changes lead to the formation of new substances with different chemical properties.
Physical changes refer to transformations in matter that do not result in the formation of new substances. Instead, they only involve alterations in the physical properties of matter, such as its shape, size, phase, or state of aggregation. Physical changes can be reversible or irreversible, depending on the conditions. Examples of physical changes include melting, freezing, boiling, condensation, sublimation, and dissolving. These transformations do not involve a change in the chemical composition of the substances involved.
Chemical changes, on the other hand, involve transformations in matter that result in the formation of new substances with different chemical properties. These changes occur at the molecular level and involve the breaking and forming of chemical bonds. Chemical changes are often accompanied by a release or absorption of energy, such as heat, light, or sound. Some examples of chemical changes include burning, rusting, fermentation, and digestion. These transformations involve a rearrangement of atoms and a change in the chemical composition of the substances involved.
In conclusion, physical and chemical changes in chemistry are distinct types of transformations that matter can undergo. Physical changes only affect the physical properties of substances, while chemical changes involve the formation of new substances with different chemical properties. Understanding and identifying these changes is essential in various scientific fields, including materials science, environmental chemistry, and pharmaceutical research.
Understanding the Difference between Physical and Chemical Changes
When studying chemistry and conducting various experiments, it is crucial to understand the difference between physical and chemical changes. A physical change refers to a change in the physical properties of a substance, such as its shape, size, or state of matter, without altering its chemical composition. On the other hand, a chemical change involves the formation of new substances with different chemical properties than the original substances.
One way to differentiate between physical and chemical changes is by observing if there are any changes in energy during the process. Physical changes usually do not involve the absorption or release of energy, while chemical changes often accompany an energy exchange, such as heat being released or absorbed. Additionally, physical changes are usually reversible, meaning that the original substance can be restored, whereas chemical changes are often irreversible.
During a physical change, the particles of a substance may be rearranged, but the composition of the molecules remains the same. For example, when water freezes, its particles arrange in a regular pattern, but the molecules remain H2O. In contrast, during a chemical change, the bonds between atoms are broken and new bonds are formed, resulting in the creation of different substances with unique chemical properties.
In conclusion, understanding the distinction between physical and chemical changes is crucial in the field of chemistry. Physical changes involve alterations in physical properties without changing the chemical composition, while chemical changes result in the formation of new substances with different chemical properties. By being able to identify and differentiate between these types of changes, scientists can better understand the behavior and transformations of various substances.
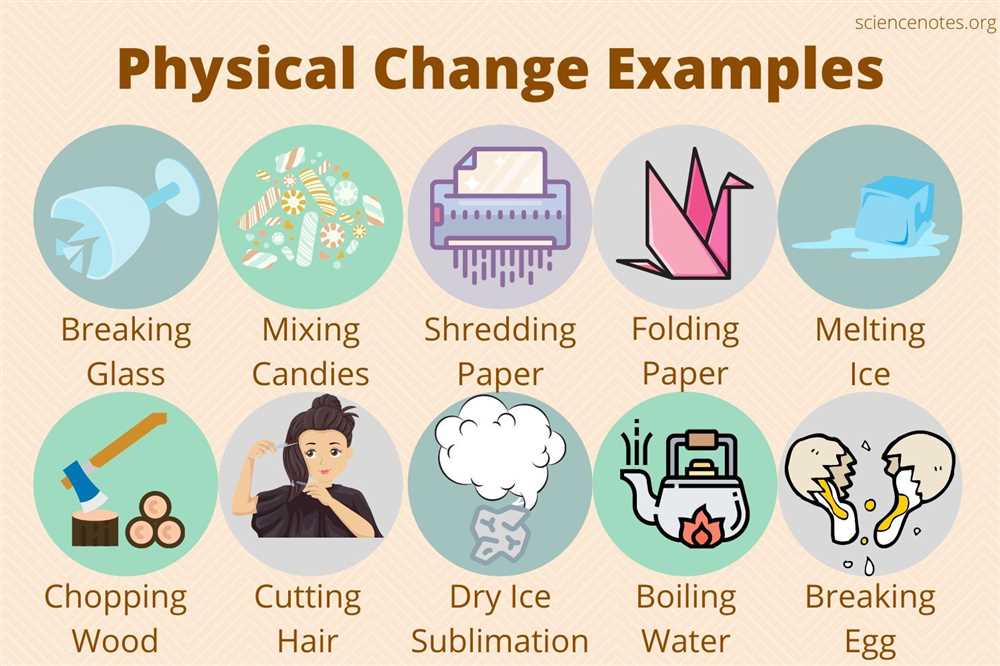
Examples of Physical Changes

A physical change is a type of change in which the appearance or physical properties of a substance are altered, but the chemical composition remains the same. This means that the substance can be changed back to its original form without any chemical reactions occurring. There are several common examples of physical changes that occur in everyday life.
One example of a physical change is when water changes from a solid state (ice) to a liquid state (water) when heated. This is known as melting. The appearance of the water changes from a solid to a liquid, but the chemical composition of H2O remains the same. Similarly, when liquid water is cooled, it changes back into its solid state (ice), which is known as freezing.
Another example of a physical change is when a piece of paper is torn into smaller pieces. The appearance of the paper changes, but it is still composed of the same chemical substances that make up paper. This type of change is known as tearing or shredding.
In addition, when a magnet attracts and separates iron filings from a mixture of iron and sand, it is an example of a physical change. The iron filings can be easily separated from the mixture using the magnetic force. The appearance and physical properties of the iron filings change, but the chemical composition of iron remains the same.
These examples illustrate how physical changes can occur in different forms. Whether it is the change in state of a substance, the tearing of a material, or the separation of components in a mixture, physical changes play a significant role in our everyday lives.
Examples of Chemical Changes
Chemical changes occur when substances undergo a transformation that results in the formation of new substances with different chemical properties. Here are a few examples of chemical changes:
- Rusting of iron: When iron is exposed to oxygen and moisture, it undergoes a chemical reaction known as rusting. The iron reacts with oxygen to form iron oxide, a new substance with different properties.
- Burning of wood: When wood is burned, it undergoes a chemical change known as combustion. The complex organic molecules in wood break down into simpler molecules, such as carbon dioxide and water vapor.
- Formation of rust stains on clothing: When clothes come into contact with iron-containing water or metal, a chemical reaction may occur, resulting in the formation of rust stains. This is an example of a chemical change that alters the appearance of the clothing.
- Digestion of food: The process of digestion involves the breaking down of complex molecules in food into simpler molecules that can be absorbed by the body. Enzymes in the digestive system catalyze chemical reactions during digestion, resulting in the transformation of food into nutrients.
- Combustion of fossil fuels: When fossil fuels, such as gasoline, coal, or natural gas, are burned, they undergo a chemical reaction that releases energy. This is a chemical change that transforms the potential energy stored in the fuel into heat and other forms of energy.
These examples illustrate the diverse range of chemical changes that occur in our everyday lives. Whether it is the formation of rust or the digestion of food, chemical changes play a fundamental role in shaping the world around us.
Conducting a Physical and Chemical Changes Lab
The purpose of conducting a physical and chemical changes lab is to explore the different ways in which matter can change. This lab allows students to observe and analyze the physical and chemical properties of various substances, and to identify the types of changes that occur. By conducting this lab, students can develop a better understanding of the differences between physical and chemical changes, and how these changes can affect the properties and behavior of matter.
One of the key steps in conducting a physical and chemical changes lab is selecting the right materials and substances to test. It is important to choose a variety of substances that exhibit different physical and chemical properties, such as solids, liquids, and gases. This ensures that students have a wide range of observations and data to analyze. Additionally, it is important to provide students with clear instructions and safety guidelines to follow, in order to ensure the lab is conducted safely and accurately.
- Materials: The materials needed for this lab may include test tubes, beakers, droppers, various substances (e.g. iron filings, baking soda, vinegar), and safety equipment such as goggles and gloves.
- Procedure: The lab can be conducted by following a step-by-step procedure. This may include adding different substances to test tubes and observing the changes that occur, recording the observations in a lab notebook, and analyzing the results. Students can also perform tests such as heating, cooling, and mixing substances to observe their effects on the changes that occur.
Once the lab is completed, students can compile their data and observations into a lab report or presentation. This allows them to share their findings and conclusions with their classmates and the teacher. It is important to encourage students to critically analyze their data, draw connections between the observed changes and the properties of the substances, and explain the underlying scientific principles that explain the observed changes.
In conclusion, conducting a physical and chemical changes lab is an engaging and hands-on way for students to explore the properties of matter and understand the differences between physical and chemical changes. By carefully selecting materials, following a structured procedure, and analyzing their observations, students can develop a better understanding of these concepts and enhance their scientific inquiry skills.
Before conducting any experiment, scientists formulate hypotheses based on their knowledge and observations. In the context of physical and chemical changes, hypotheses can be made about the behavior of different substances when exposed to various conditions.
Hypotheses

- When a solid substance is heated, it will melt and become a liquid.
- When a liquid substance is cooled, it will solidify and become a solid.
- When a liquid substance is heated, it will evaporate and become a gas.
- When a gas substance is cooled, it will condense and become a liquid.
- Chemical reactions may occur when two or more substances are mixed together.
These hypotheses provide a basis for designing experiments to investigate physical and chemical changes. The next step is to set up the experimental apparatus and determine the variables that will be measured.
For the lab on physical and chemical changes, the experimental setup may involve various materials and equipment. Some possible components of the setup include:
- Various substances, such as solids, liquids, and gases
- Heating sources, such as Bunsen burners or hot plates
- Thermometers or temperature probes to measure temperature changes
- Glassware like beakers, flasks, or test tubes for mixing substances
- Balance scales or mass measuring devices to measure the mass of substances
- Chemical indicators or litmus paper to test for the presence of certain substances
The experimental setup should be carefully planned to ensure accurate and reliable results. It is important to control variables, such as the amount of substance used, the temperature, and the time of observation. By following a systematic approach and recording observations, scientists can test their hypotheses and draw conclusions about physical and chemical changes.