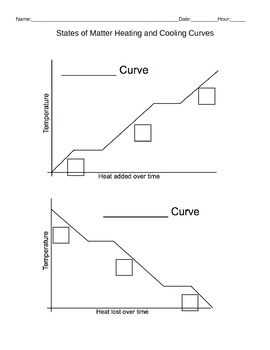
In the world of thermodynamics, understanding the physical behavior of matter during the processes of heating and cooling is crucial. Heating and cooling curves provide valuable insights into how a substance changes as it gains or loses heat. These curves are essential in determining the specific heat capacity of a substance, as well as its phase changes.
When a substance is heated, its temperature increases, causing its particles to move more quickly. This increase in particle motion results in an expansion of the substance and a rise in temperature. The heating curve captures this relationship between heat added to a substance and its temperature. It shows a steady increase in temperature as heat is continuously added to the substance. The slope of the heating curve is related to the substance’s specific heat capacity, which quantifies how much heat energy is required to raise the temperature of a given amount of the substance.
Cooling, on the other hand, involves the removal of heat energy from a substance, causing its temperature to decrease. As the substance loses heat, its particles slow down, and the substance contracts. The cooling curve demonstrates this relationship between heat removal and temperature decrease. It shows a steady decrease in temperature as heat is continuously removed from the substance. The slope of the cooling curve is related to the substance’s specific heat capacity as well, but in this case, it represents how much heat energy is released during the cooling process.
Heating and cooling curves also display important information about phase changes, such as melting and evaporation. During these phase transitions, the temperature of a substance remains constant even though heat is being added or removed. This is because the heat energy is being used to break or form intermolecular bonds, rather than increase or decrease the kinetic energy of the individual particles. The flat sections in the heating and cooling curves correspond to these phase changes, providing insight into the energy required for these transformations.
In conclusion, heating and cooling curves are powerful tools in understanding the physical behavior of matter. They showcase the relationship between heat added or removed and changes in temperature, as well as provide valuable information about the specific heat capacity and phase changes of a substance. By analyzing these curves, scientists can gain a deeper understanding of the thermodynamics involved in heating and cooling processes, allowing for the development of more efficient heating and cooling systems.
Understanding matter and its physical behavior

A heating curve shows how the temperature of a substance changes as heat is added to it, while a cooling curve shows how the temperature changes as heat is removed from the substance. These curves can provide valuable information about the energy transfer and phase changes that occur during heating or cooling. It is important to note that during phase changes, the temperature of the substance remains constant even though heat is still being added or removed.
In order to interpret these curves, it is necessary to understand the concept of latent heat. Latent heat is the energy required to change the state of a substance without changing its temperature. For example, during the melting process, heat is added to the solid substance to break the bonds between the particles and change it into a liquid state. The energy absorbed during this process is known as the latent heat of fusion.
The heating and cooling curves also demonstrate the concept of specific heat capacity. Specific heat capacity is the amount of heat required to raise the temperature of a substance by a certain amount. Different substances have different specific heat capacities, which is why some materials heat up or cool down faster than others.
In conclusion, understanding matter and its physical behavior involves studying the heating and cooling curves, as well as considering concepts such as latent heat and specific heat capacity. These curves provide insights into the energy transfer and phase changes that occur during heating or cooling, and help us comprehend the behavior of matter under different conditions.
The concept of heating curves
A heating curve is a graphical representation of the changes in temperature and phase transitions that occur as a substance is heated. It shows the relationship between the amount of heat applied to a substance and its corresponding temperature. Heating curves are commonly used in physics and chemistry to analyze and understand the behavior of matter during the heating process.
When a substance is heated, its temperature increases until it reaches its melting point. At this point, the substance undergoes a phase transition known as melting, where it changes from a solid to a liquid state. During this phase transition, the temperature remains constant, as the heat energy is being used to break the intermolecular forces holding the solid particles together.
Once the substance has completely melted, the temperature begins to increase again until it reaches its boiling point. At this point, another phase transition occurs, known as boiling, where the substance changes from a liquid to a gaseous state. During this phase transition, the temperature again remains constant, as the heat energy is being used to overcome the intermolecular forces holding the liquid particles together.
The heating curve can also show other phase transitions, such as sublimation and condensation, depending on the substance being heated. By analyzing the heating curve, scientists can determine the melting and boiling points of a substance, as well as its heat capacity, which is the amount of heat required to raise the temperature of a substance by a certain amount.
Factors affecting heating curves
Heating curves represent the change in temperature of a substance as it is heated over time. Several factors can affect the shape and pattern of a heating curve, including the nature of the substance being heated, the amount of heat being applied, and the surrounding environment.
Nature of the substance
The physical and chemical properties of the substance being heated play a significant role in determining its heating curve. Different substances have different melting and boiling points, which can result in variations in the temperature at which changes in phase occur. For example, substances with high boiling points will require more heat to reach their boiling point, resulting in a steeper slope on the heating curve.
The specific heat capacity of a substance also affects its heating curve. Substances with higher specific heat capacities require more heat to raise their temperature, resulting in a slower rate of temperature increase and a flatter slope on the heating curve.
Amount of heat applied
The amount of heat being applied to the substance also affects its heating curve. Increasing the heat input will result in a faster rate of temperature increase and a steeper slope on the heating curve. Conversely, decreasing the heat input will result in a slower rate of temperature increase and a flatter slope.
Surrounding environment
The surrounding environment can have an impact on the shape of the heating curve. If the substance is heated in an environment with good thermal conductivity, such as a metal container, heat will be transferred more efficiently, resulting in a faster rate of temperature increase and a steeper slope on the heating curve. Conversely, if the substance is heated in an environment with poor thermal conductivity, such as a vacuum, heat transfer will be less efficient, resulting in a slower rate of temperature increase and a flatter slope.
In conclusion, the nature of the substance, the amount of heat applied, and the surrounding environment all play a role in determining the shape and pattern of a heating curve. Understanding these factors is essential for predicting and interpreting the behavior of matter during heating processes.
Interpretation of heating curves
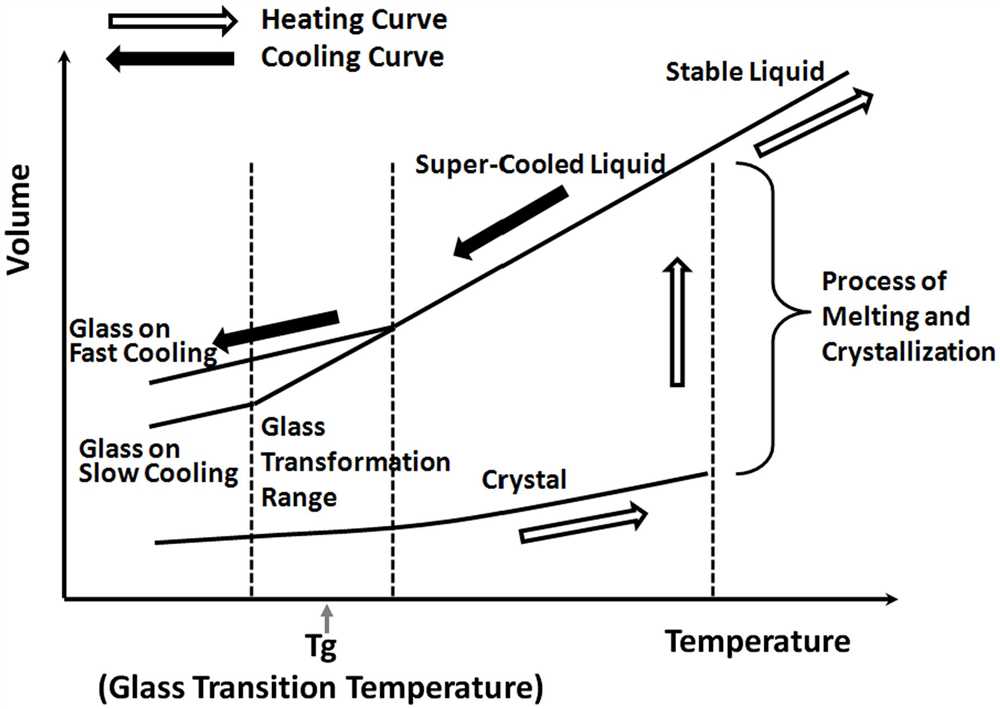
Heating curves are graphical representations of the relationship between temperature and time during the process of heating a substance. By analyzing heating curves, we can gain valuable insights into the properties and behavior of different substances.
One key observation we can make from a heating curve is the presence of plateaus. These plateaus represent phase changes, where the substance undergoes a transition from one phase to another, such as solid to liquid or liquid to gas. During these phase changes, the temperature remains constant even though heat is being added. This is because the heat energy is used to break the intermolecular forces holding the molecules together, rather than increasing the average kinetic energy of the molecules.
Another important observation from a heating curve is the slope of the line segments. The steepness of a line segment indicates the rate at which the substance is heating up. A steeper slope indicates a faster rate of temperature increase, while a less steep slope suggests a slower rate. From this information, we can compare the heating rates of different substances and determine which one heats up more quickly or slowly.
Heating curves also provide information about the specific heat capacity of a substance. The specific heat capacity is the amount of energy required to raise the temperature of a unit mass of the substance by one degree Celsius. By analyzing the trends in temperature change during different segments of the heating curve, we can calculate the specific heat capacity of the substance, which is an important property for various applications in physics and chemistry.
The concept of cooling curves
Cooling curves are graphical representations of how the temperature of a substance changes over time as it cools down. These curves are obtained by measuring the temperature of the substance at regular intervals and plotting the data points on a graph. They provide valuable information about the physical behavior of matter during the cooling process.
When a substance is heated and then allowed to cool, its temperature decreases gradually. This decrease in temperature occurs due to the transfer of heat energy from the substance to its surroundings. Cooling curves allow us to observe and analyze this cooling process in detail.
A typical cooling curve consists of several distinct regions. Initially, when the substance is still at a high temperature, the curve is steep, indicating a rapid decrease in temperature. As time progresses and the substance continues to lose heat, the curve gradually becomes flatter, indicating a slower rate of cooling.
The plateau region of the cooling curve is particularly interesting. During this phase, the substance undergoes a phase change, such as solidification or condensation, and its temperature remains constant even though heat is being lost. This region of the curve is typically horizontal, denoting a constant temperature.
The interpretation of cooling curves can provide insights into various properties of substances. For example, the slope of the curve at different points can be used to determine the rate of cooling. The length of the plateau region can indicate the amount of heat required for a phase change. Additionally, the shape and characteristics of the cooling curve can help identify the substance being cooled.
Factors Affecting Cooling Curves
The cooling curve of a substance is influenced by several factors that affect its physical behavior during the cooling process. Understanding these factors is crucial in various applications, such as materials science, thermodynamics, and industrial processes. Here are some key factors that impact cooling curves:
1. Initial temperature: The starting temperature of the substance significantly affects the cooling curve. The higher the initial temperature, the longer it takes for the substance to cool down to a specific temperature. This is due to the greater amount of thermal energy that needs to be dissipated.
2. Heat transfer medium: The medium through which heat is transferred from the substance also plays a role in determining the cooling curve. Different materials have different thermal conductivities, which affect how efficiently heat is transferred. For example, substances cooled in air will have different cooling curves compared to those cooled in water.
3. Heat capacity: The heat capacity of a substance determines how much thermal energy it can store. Substances with higher heat capacities require more time and energy to cool down, resulting in a longer cooling curve. This property is particularly important in applications where rapid cooling is desired, such as in the production of metals or temperature-sensitive materials.
4. Surface area: The surface area of the substance in contact with the surroundings affects the rate of heat dissipation and, consequently, the cooling curve. Larger surface areas allow for more efficient heat transfer, resulting in faster cooling rates. This is why objects like thin plates or films cool down faster compared to massive objects of the same material.
5. Ambient temperature: The temperature of the surroundings also influences the cooling curve. If the ambient temperature is higher than the substance’s final desired temperature, the cooling process will be slower. On the other hand, if the ambient temperature is lower, the cooling process can be faster, resulting in a steeper cooling curve.
Understanding and controlling these factors can help optimize processes involving cooling curves, leading to more efficient cooling and better control of material properties.
Interpretation of cooling curves

The interpretation of cooling curves plays a crucial role in understanding the physical behavior of matter during the cooling process. Cooling curves provide valuable information about the temperature changes and the associated phase transitions that occur as a substance cools down.
At the start of the cooling process, the temperature is typically high and the substance is in the liquid phase. As heat is gradually removed, the substance begins to cool and its temperature decreases. The cooling curve shows a gradual decline in temperature as energy is transferred from the substance to its surroundings.
As the temperature continues to decrease, the substance undergoes phase transitions. These phase transitions are marked by characteristic changes in the cooling curve. For example, when a liquid substance reaches its freezing point, its temperature remains constant as the substance changes from a liquid to a solid state. This plateau in the cooling curve corresponds to the latent heat of fusion, which is the energy required for the substance to change from a liquid to a solid.
In some cases, multiple phase transitions can occur during the cooling process. These can be observed as multiple plateaus or flat sections in the cooling curve. Each plateau corresponds to a different phase transition, such as the solidification of a liquid or the crystallization of a supercooled substance.
By analyzing the cooling curve, scientists and researchers can determine important properties of a substance, such as its melting point, freezing point, and heat of fusion. The cooling curve provides a visual representation of how temperature changes over time during the cooling process, allowing for a better understanding of the physical behavior of matter.