
If you’re preparing for your physical science final exam, you’ve come to the right place. This study guide will help you review the key concepts and topics covered in your course, allowing you to confidently tackle any questions that may come your way.
Physical science is the study of the natural world and the laws that govern it. It encompasses a wide range of topics, including physics, chemistry, and earth science. In this study guide, we will cover the main principles and theories in each of these fields, as well as provide examples and practice problems to help you solidify your understanding.
One of the key topics in physical science is Newton’s laws of motion. These laws describe the relationship between the motion of an object and the forces acting upon it. Understanding these laws will allow you to analyze and predict the behavior of objects in various scenarios, such as when they are in motion or at rest.
Another important concept in physical science is the periodic table of elements. This table organizes all known elements based on their atomic structure and properties. By familiarizing yourself with the periodic table, you will be able to identify elements, understand their properties, and determine how they interact and form compounds.
Overview of the Physical Science Final Exam
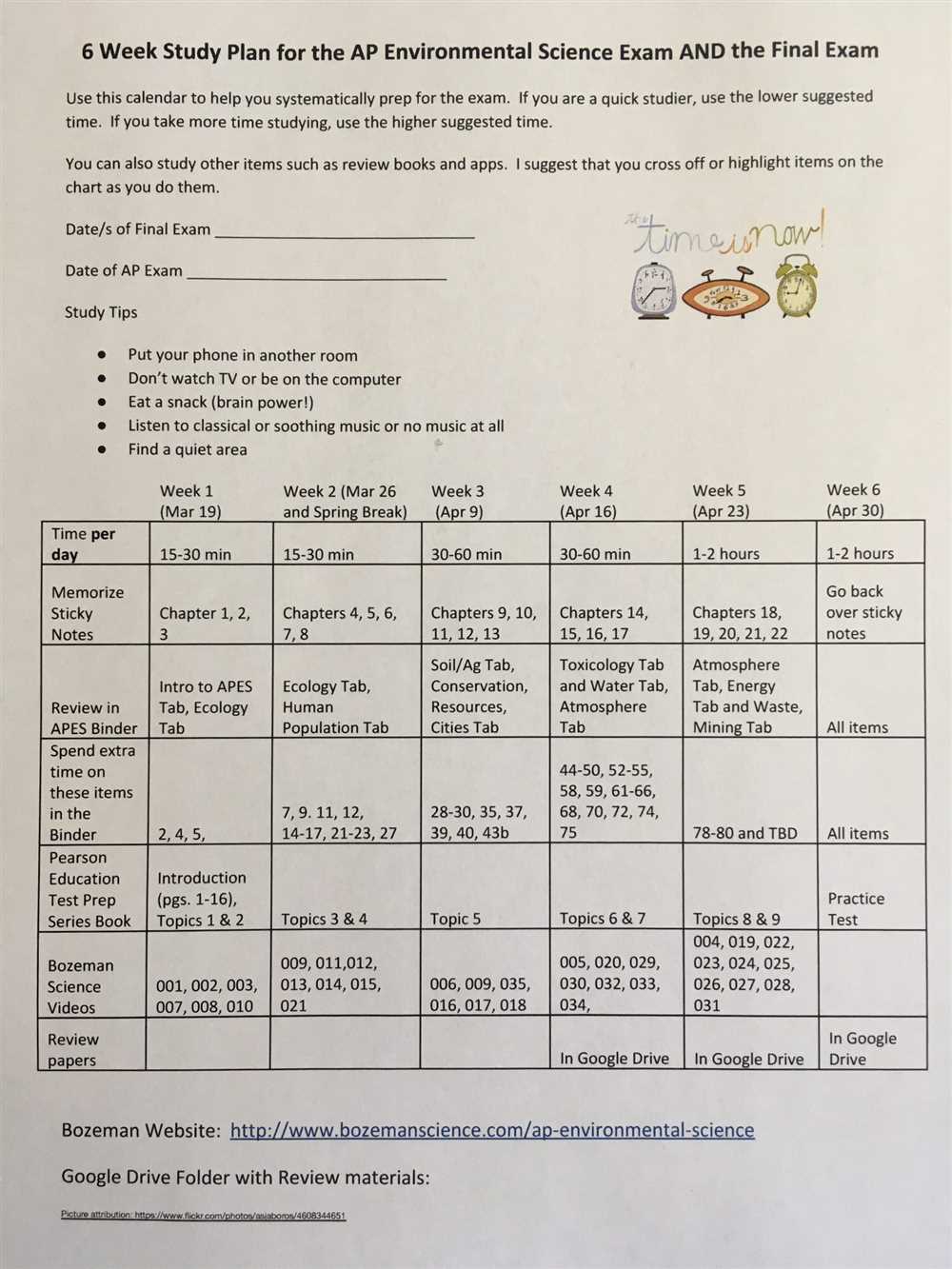
The Physical Science Final Exam is the culminating assessment for the course and covers a wide range of topics in the field of physical science. This exam is designed to test your knowledge and understanding of key concepts, theories, and principles that have been explored throughout the semester. It will assess your ability to apply these concepts to real-world situations and solve problems using scientific methods.
The exam will consist of multiple-choice questions, short-answer questions, and possibly some practical application questions. The multiple-choice questions will require you to select the correct answer from a set of options. The short-answer questions will ask you to provide brief explanations or definitions of scientific terms or concepts. The practical application questions will assess your ability to apply scientific knowledge and principles to real-world scenarios.
To prepare for the exam, it is important to review all of the material covered in the course. This includes topics such as matter and energy, forces and motion, waves and sound, electricity and magnetism, and the basics of chemistry and physics. It is also beneficial to practice solving problems and answering questions similar to those that may appear on the exam. This will help you become more familiar with the format and improve your problem-solving skills.
During the exam, be sure to read each question carefully and fully understand what is being asked before selecting your answer or providing a response. Take your time and allocate your time wisely, as there will be a limited amount of time to complete the exam. Use any equations or formulas that have been provided to you, and show your work and reasoning when applicable.
Overall, the Physical Science Final Exam is an opportunity for you to demonstrate your understanding and application of physical science concepts. By reviewing the course material, practicing problem-solving, and being prepared, you can approach the exam with confidence and increase your chances of success.
Important Concepts and Theories in Physical Science

In the field of physical science, there are several important concepts and theories that form the foundation of our understanding of the natural world. These concepts and theories help us explain and predict the behavior of matter and energy. One of the key concepts in physical science is the law of conservation of energy, which states that energy can neither be created nor destroyed, but only transferred or transformed. This concept is fundamental to understanding processes such as energy transfer in chemical reactions and the conversion of energy from one form to another.
Another important concept in physical science is Newton’s laws of motion. These laws, formulated by Sir Isaac Newton, describe the relationship between the motion of an object and the forces acting upon it. Newton’s first law, also known as the law of inertia, states that an object will remain at rest or in uniform motion in a straight line unless acted upon by an external force. His second law relates the acceleration of an object to the force applied to it and its mass, while his third law states that for every action, there is an equal and opposite reaction.
- Law of conservation of energy
- Newton’s laws of motion
- Electromagnetic spectrum
- Thermodynamics and heat transfer
- Atomic theory and periodic table
Other important concepts in physical science include the electromagnetic spectrum, which describes the range of electromagnetic waves, from radio waves to gamma rays, and their properties. Understanding the electromagnetic spectrum is crucial in fields such as telecommunications, astronomy, and imaging technologies. Similarly, thermodynamics and heat transfer play a significant role in the study of energy and its transformation in various systems. These concepts are essential in fields such as engineering, environmental science, and chemistry.
Finally, the atomic theory and periodic table are fundamental to the study of chemistry and the understanding of the building blocks of matter. The atomic theory postulates that matter is composed of indivisible particles called atoms, and the periodic table organizes these atoms based on their properties and atomic structure. This concept is key to understanding chemical reactions, the behavior of elements, and the formation of compounds.
In summary
Physical science encompasses a wide range of concepts and theories. The concepts of conservation of energy, Newton’s laws of motion, the electromagnetic spectrum, thermodynamics and heat transfer, and the atomic theory and periodic table are among the important foundations of this field. Understanding these concepts allows us to explain and predict the behavior of matter and energy in various systems and has practical applications in many fields of science and technology.
Laws of Motion
The laws of motion, developed by Sir Isaac Newton, are fundamental principles in physics that explain the behavior of objects in motion. These laws provide a framework for understanding how forces and motion interact with each other.
1. Newton’s First Law of Motion: Also known as the law of inertia, this law states that an object at rest will stay at rest, and an object in motion will continue in motion with a constant velocity, unless acted upon by an external force. Essentially, an object will maintain its current state of motion unless a force acts upon it.
2. Newton’s Second Law of Motion: This law states that the acceleration of an object is directly proportional to the net force acting on it and inversely proportional to its mass. The formula for this law is F = ma, where F is the net force applied to an object, m is its mass, and a is the resulting acceleration.
3. Newton’s Third Law of Motion: This law states that for every action, there is an equal and opposite reaction. This means that when one object exerts a force on another object, the second object exerts a force of equal magnitude but in the opposite direction on the first object. In other words, all forces occur in pairs.
These laws of motion play a crucial role in understanding and predicting the motion of objects in various scenarios. They have countless practical applications, from explaining how vehicles move to understanding the behavior of planets in orbit.
- For example, Newton’s first law of motion helps explain why passengers in a car lurch forward when the car suddenly stops. The inertia of the passengers causes them to resist changes in their state of motion, leading to this phenomenon.
- Newton’s second law of motion is often used in calculations involving force, mass, and acceleration. It allows engineers to design structures and machines, taking into account the forces and accelerations involved.
- Newton’s third law of motion can be observed in various daily situations. For instance, when a person pushes against a wall, the wall pushes back with an equal force in the opposite direction. This is why the person can feel the resistance of the wall.
In conclusion, the laws of motion provide the foundation for understanding the physics of motion and force. By applying these laws, scientists and engineers can analyze and predict the behavior of objects in various scenarios, enabling advancements in technology and our understanding of the world around us.
Conservation of Energy

The law of conservation of energy states that energy cannot be created or destroyed, but it can be transformed from one form to another. This principle is fundamental in understanding the behavior and interactions of objects in the physical world.
Kinetic energy is the energy possessed by an object due to its motion. The formula for calculating kinetic energy is K = ½ mv², where K is the kinetic energy, m is the mass of the object, and v is its velocity. According to the law of conservation of energy, the total amount of kinetic energy in a system remains constant as long as there is no external force acting on it.
Potential energy is the energy possessed by an object due to its position or condition. There are different types of potential energy, such as gravitational potential energy and elastic potential energy. Gravitational potential energy is the energy an object has due to its position in a gravitational field, and it is given by the formula PE = mgh, where PE is the potential energy, m is the mass, g is the acceleration due to gravity, and h is the height. Elastic potential energy is the energy stored in a stretched or compressed elastic object, and it is given by the formula PE = ½ kx², where k is the spring constant and x is the displacement from the equilibrium position.
The law of conservation of energy states that the total energy of a closed system remains constant over time. This means that energy cannot be created or destroyed, but it can be converted from one form to another. For example, if a ball is dropped from a certain height, its potential energy is converted into kinetic energy as it falls. Similarly, if a moving car comes to a stop, its kinetic energy is converted into other forms of energy, such as heat and sound.
- The law of conservation of energy is applicable to all types of energy, including mechanical energy, thermal energy, electrical energy, and chemical energy.
- Energy transformations can occur within a system or between different systems.
- The efficiency of energy transformations in real-world systems is less than 100%, meaning that some energy is always lost as waste heat.
Understanding the principles of conservation of energy is crucial for solving problems related to motion, calculations of energy transformations, and designing efficient systems that minimize energy losses. It provides a foundation for explaining the behavior of objects and phenomena observed in the physical world.
Atomic Structure
The study of atomic structure is crucial in understanding the fundamental building blocks of matter. Atoms are the smallest units of an element that retain its chemical properties. They consist of subatomic particles, including protons, neutrons, and electrons, which are organized in a specific way within the atom.
Electrons are negatively charged particles that orbit around the nucleus of an atom in energy levels, or shells. They have a negligible mass compared to protons and neutrons, but their behavior determines the chemical properties of an element. The number of electrons in an atom is equal to the number of protons, creating a neutral charge.
Protons are positively charged particles located in the nucleus of an atom. They have a relative mass of 1 and a charge of +1. The number of protons in an atom determines the element’s atomic number, which identifies the element on the periodic table.
Neutrons are neutral particles located in the nucleus of an atom. They have a relative mass of 1, similar to protons, but no electric charge. The presence of neutrons affects the stability and isotopes of an element.
The arrangement of these subatomic particles results in the overall structure of an atom. The nucleus, composed of protons and neutrons, is at the center, while electrons occupy specific energy levels around it. The number of protons and electrons determines the overall charge of the atom, which can be positive, negative, or neutral.
Understanding the atomic structure is crucial in predicting the chemical behavior of elements and compounds. It allows scientists to explain phenomena such as bonding, reactivity, and the formation of ions. The discovery and understanding of atomic structure have revolutionized our understanding of the physical world and have contributed to advancements in various fields, including chemistry, physics, and materials science.
Chemical Reactions and Equations
A chemical reaction is a process in which one or more substances are transformed into new substances with different properties. These reactions occur when atoms rearrange their bonds to form new molecules. Chemical reactions can be represented by balanced chemical equations, which show the reactants on the left side and the products on the right side of the arrow.
Types of Chemical Reactions:
- Combination Reactions: In these reactions, two or more substances combine to form a single product. The general form of a combination reaction is A + B → AB.
- Decomposition Reactions: In these reactions, a single compound breaks down into two or more simpler substances. The general form of a decomposition reaction is AB → A + B.
- Displacement (Replacement) Reactions: In these reactions, an element or ion in a compound is replaced by another element or ion. The general form of a displacement reaction is A + BC → AC + B.
- Double Displacement (Metathesis) Reactions: In these reactions, the cations and anions of two compounds exchange places, resulting in the formation of new compounds. The general form of a double displacement reaction is AB + CD → AD + CB.
- Combustion Reactions: In these reactions, a substance reacts with oxygen to produce carbon dioxide and water, releasing a large amount of heat. The general form of a combustion reaction is fuel + oxygen → carbon dioxide + water.
It is important to balance chemical equations in order to satisfy the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. By ensuring that the number of atoms of each element is the same on both sides of the equation, we can show that mass is conserved.