
The Pogil Electron Configuration and Orbitals Answer Key Model 1 is a valuable tool for understanding the electron configuration and orbital arrangement of atoms. This model allows students to explore the distribution of electrons within an atom’s energy levels and sublevels, helping them grasp the concept of electron configuration.
By using the Pogil Electron Configuration and Orbitals Answer Key Model 1, students can visualize the different energy levels and shapes of the orbitals. This hands-on approach enhances their understanding of how electrons occupy specific orbitals based on their energy and the Pauli exclusion principle.
The answer key model 1 provides students with a step-by-step guide to determining the electron configuration of various elements. It helps them interpret the periodic table and understand the arrangement of electrons within shells and subshells. This knowledge is crucial for predicting the reactivity and chemical behavior of elements.
Overall, the Pogil Electron Configuration and Orbitals Answer Key Model 1 is an effective tool for teaching electron configuration and orbital arrangement. It empowers students to develop a deeper understanding of atomic structure and lays the foundation for further exploration in chemistry and quantum mechanics.
Pogil Electron Configuration and Orbitals Answer Key Model 1
In the Pogil Electron Configuration and Orbitals Answer Key Model 1, we explore the concept of electron configuration and orbitals in chemistry. Electron configuration refers to the arrangement of electrons in an atom. It helps to determine the behavior and properties of elements. Orbitals, on the other hand, are the regions in an atom where electrons are most likely to be found.
The answer key model 1 provides a set of questions and calculations to help students understand the relationship between electron configuration and orbitals. It covers topics such as the Aufbau principle, Hund’s rule, and the Pauli exclusion principle. These principles govern how electrons fill different energy levels and orbitals in an atom.
The Pogil model 1 answer key also includes diagrams and tables to visually represent the electron configurations and orbitals of various elements. It allows students to practice identifying the number of electrons in each energy level and the electron distributions in different orbitals.
- Question 1: What is the electron configuration of carbon?
- Question 2: How many valence electrons does oxygen have?
- Question 3: What is the electron configuration of magnesium?
By using the answer key model 1, students can check their understanding of electron configuration and orbitals and further enhance their knowledge in chemistry. It serves as a helpful tool for self-study and classroom practice.
Overview of Electron Configuration
Electron configuration refers to the arrangement of electrons in an atom or molecule. It provides important information about the energy levels and orbitals that electrons occupy. Understanding electron configuration is crucial in predicting and explaining the chemical behavior of elements.
The electron configuration of an atom is represented by a series of numbers and letters, with each number corresponding to a specific energy level and each letter representing a specific orbital within that energy level. The arrangement follows specific rules, such as the Aufbau principle, the Pauli exclusion principle, and Hund’s rule.
The Aufbau principle states that electrons fill the lowest energy levels first before moving to higher energy levels. This leads to a specific order of filling the orbitals, with the s orbital being filled before the p orbital, followed by the d and f orbitals. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons, with opposite spins. Hund’s rule states that electrons will occupy separate orbitals within the same energy level before pairing up.
The electron configuration can be represented in various ways, including the spdf notation and the orbital diagram. The spdf notation uses letters to represent the type of orbital and superscripts to indicate the number of electrons in each orbital. The orbital diagram represents the energy levels as horizontal lines, with the orbitals as boxes used to represent the electrons.
Overall, electron configuration plays a vital role in understanding the behavior of atoms and molecules. It provides a framework for explaining the periodic trends, reactivity, and bonding properties of elements. By studying electron configuration, scientists are able to make predictions about the chemical properties of different elements and their compounds.
Understanding Orbitals
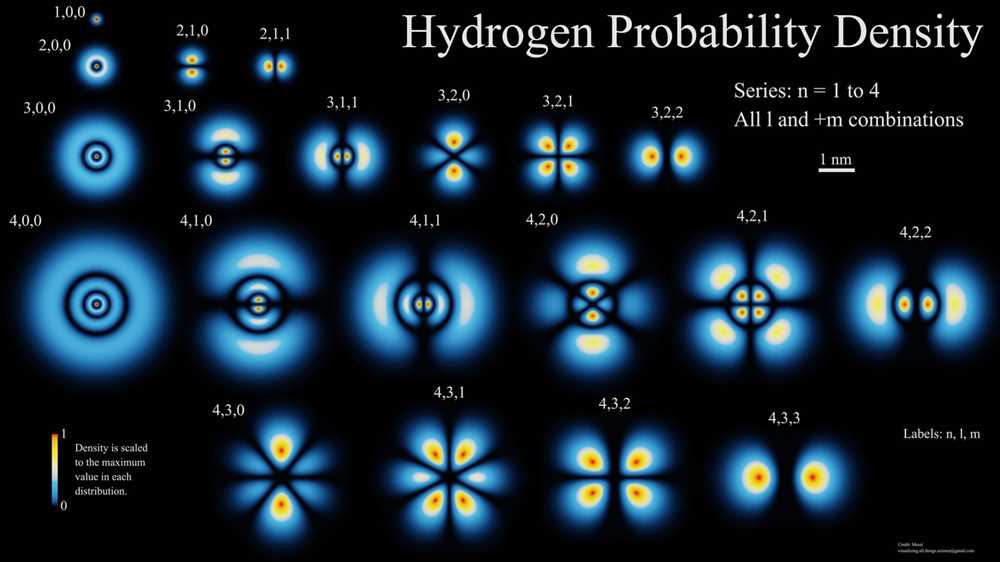
Orbitals are a fundamental concept in the field of quantum mechanics and play a crucial role in understanding the behavior of electrons in atoms. These regions of space around the nucleus indicate the probability of finding an electron. The arrangement of electrons in orbitals determines the electron configuration of an atom, which in turn determines its chemical properties.
There are four types of orbitals: s, p, d, and f. Each orbital has a specific shape and can hold a maximum number of electrons. The s orbital is spherical and can hold up to 2 electrons, while the p orbital is dumbbell-shaped and can hold up to 6 electrons. The d and f orbitals have more complex shapes and can hold up to 10 and 14 electrons, respectively.
The orbitals are organized into energy levels or shells, with each shell containing one or more subshells. The energy level corresponds to the distance of the orbital from the nucleus, with higher energy levels located farther away. The subshells are labeled using the letters s, p, d, and f, corresponding to the four types of orbitals.
Electron configuration is a way to represent the arrangement of electrons in an atom using the symbols of the corresponding orbitals. For example, the electron configuration of carbon is 1s2 2s2 2p2, indicating that there are 2 electrons in the 1s orbital, 2 electrons in the 2s orbital, and 2 electrons in the 2p orbital.
Understanding orbitals is essential for studying atomic structure, chemical bonding, and predicting the behavior of elements and compounds. The concept of orbitals provides a more accurate representation of electron distribution in atoms compared to the older Bohr model, which assumed that electrons moved in fixed circular orbits around the nucleus.
The Relationship Between Electrons, Energy Levels, and Orbitals
In the field of chemistry, the behavior and arrangement of electrons in an atom play a crucial role in determining its properties and chemical reactivity. Electrons occupy specific energy levels and orbitals within an atom, and understanding their relationship is essential for understanding the behavior of atoms and molecules.
Energy levels, also known as electron shells or principal quantum numbers, are regions of space around the nucleus where electrons are most likely to be found. These energy levels are organized into specific shells, labeled with the principal quantum numbers n = 1, 2, 3, and so on. Electrons with lower energy are found closer to the nucleus in the lower energy levels, while electrons with higher energy are found in the outer shells.
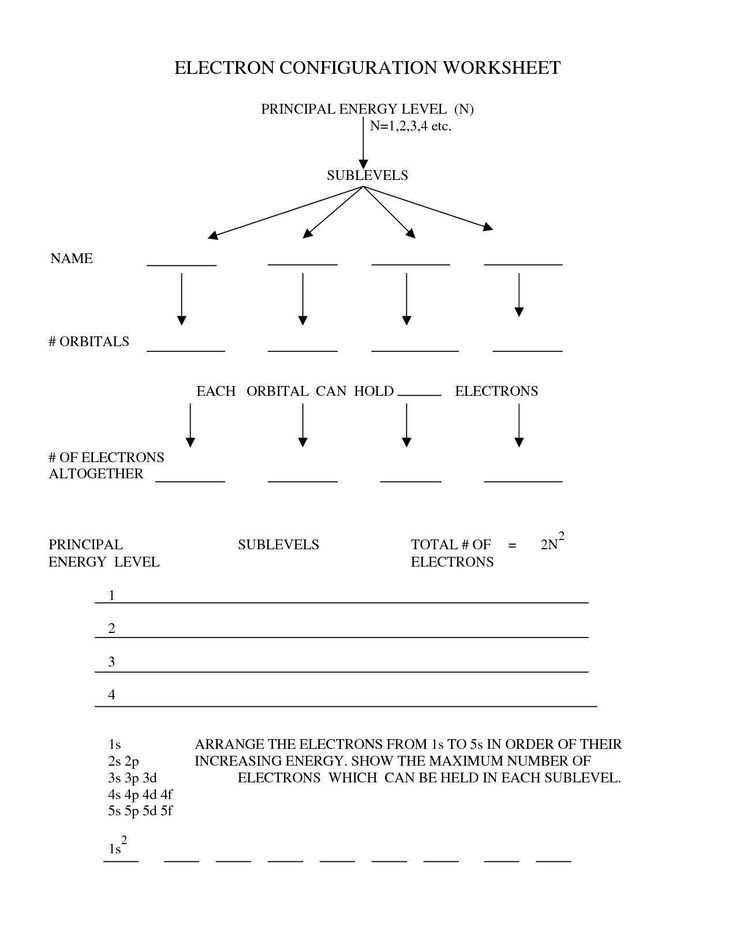
Each energy level is further divided into sublevels, also known as orbitals. Orbitals are regions within an energy level where electrons have the highest probability of being located. The different types of orbitals are labeled as s, p, d, and f, each with a specific shape and orientation within the energy level. The s orbital is spherical, while the p orbitals are dumbbell-shaped, and the d and f orbitals have more complex shapes.
The number of orbitals within an energy level depends on the principal quantum number. For example, the first energy level (n = 1) has only one s orbital, the second energy level (n = 2) has one s and three p orbitals, the third energy level (n = 3) has one s, three p, and five d orbitals, and so on. The total number of orbitals within an energy level is equal to n^2, where n is the principal quantum number.
The Aufbau Principle: Filling Orbitals in Order of Increasing Energy
One of the fundamental principles in understanding the electron configuration of an atom is the Aufbau principle. According to this principle, electrons occupy orbitals in order of increasing energy. This means that as electrons are added to an atom, they fill the available orbitals starting from the lowest energy level to the highest.
Electrons are arranged in different orbitals, which are regions of space where an electron is likely to be found. These orbitals are organized into electron shells and subshells, each with a specific energy level. The energy levels of the orbitals increase as the distance from the nucleus increases.
The Aufbau principle provides a systematic way to determine the electron configuration of an atom. By following this principle, we can predict the order in which electrons fill the available orbitals. The Aufbau principle is based on the idea that electrons occupy the lowest energy level first before moving to higher energy levels.
For example, the electron configuration of carbon is 1s2 2s2 2p2. This means that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital. The 1s orbital has the lowest energy level, followed by the 2s and 2p orbitals.
Understanding the Aufbau principle is crucial for predicting the chemical behavior of elements and explaining periodic trends. It allows chemists to determine the stability and reactivity of atoms based on their electron configurations. By knowing the order in which electrons occupy orbitals, scientists can make accurate predictions about the bonding and properties of different elements.
Applying Hund’s Rule: Maximizing Parallel Spins within Orbitals
Hund’s Rule is a principle in quantum mechanics that dictates how electrons fill orbitals within an atom. It states that when multiple orbitals of the same energy level are available, electrons will occupy separate orbitals with parallel spins before pairing up. This rule maximizes the total spin of the system.
For example, let’s consider the electron configuration of nitrogen, which has seven electrons. According to Hund’s Rule, the electrons will fill the three available p orbitals (px, py, and pz) individually before pairing up. This means that each orbital will contain one electron with the same spin, either spin-up or spin-down.
This configuration can be represented as 2p3, where the superscript 3 indicates the number of electrons in the p subshell. The three electrons are distributed across the three p orbitals with parallel spins, maximizing the total spin of the system.
In general, applying Hund’s Rule allows us to determine the electron configuration of an atom and predict the arrangement of electrons within its orbitals. By following this rule, we can understand how electrons occupy energy levels and subshells, providing insight into the chemical properties and behavior of elements.
It’s important to note that Hund’s Rule applies to atoms and their ground state electron configurations. In certain cases, excited states or ions may require deviations from this rule, but for the most part, Hund’s Rule provides a reliable framework for understanding electron distribution within an atom.
The Pauli Exclusion Principle: Restricting the Number of Electrons in an Orbital
The Pauli Exclusion Principle is a fundamental concept in quantum mechanics that governs the behavior of electrons in an atom. It states that no two electrons in an atom can have the same set of quantum numbers, which include the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (m), and the spin quantum number (s). This principle restricts the number of electrons that can occupy a given orbital within an atom.
According to the Pauli Exclusion Principle, each orbital can hold a maximum of two electrons, and these electrons must have opposite spins. This means that if one electron has a spin of +½, the other electron in the same orbital must have a spin of -½. This principle ensures that electrons in the same orbital have different quantum states and helps to maintain the stability and organization of electrons within an atom.
The restriction imposed by the Pauli Exclusion Principle plays a crucial role in determining the electron configuration of an atom. The electron configuration describes how the electrons are distributed among the various energy levels and orbitals of an atom. By following the rules dictated by the Pauli Exclusion Principle, scientists can determine the arrangement of electrons and predict the chemical behavior of elements.
In summary, the Pauli Exclusion Principle establishes that no more than two electrons can occupy a single orbital, and these electrons must have opposite spins. This principle is essential for understanding the electronic structure of atoms and provides a foundation for explaining the periodic trends and chemical properties of elements.
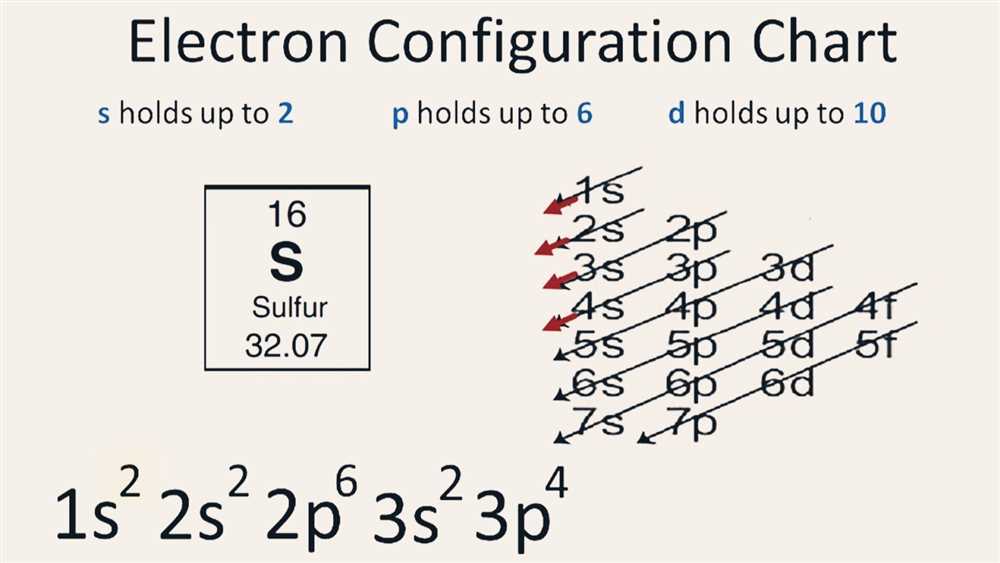
Determining Electron Configuration Using the Periodic Table
One of the key concepts in chemistry is understanding the electron configuration of an atom. Electron configuration refers to the arrangement of electrons in the energy levels and sublevels of an atom. It is crucial because it determines an atom’s chemical behavior and reactivity. Determining electron configuration can be done using the periodic table, which provides a systematic way to organize and understand the arrangement of electrons.
The periodic table is divided into rows, called periods, and columns, called groups or families. The periods represent the number of energy levels in an atom, while the groups represent elements with similar properties and similar outer electron configurations. By examining the position of an element on the periodic table, we can easily determine the electron configuration.
The electron configuration can be determined by following a few simple rules. First, electrons fill the lowest energy level before moving to higher energy levels. Second, the s sublevel can hold a maximum of 2 electrons, the p sublevel can hold a maximum of 6 electrons, the d sublevel can hold a maximum of 10 electrons, and the f sublevel can hold a maximum of 14 electrons. Third, electrons fill orbitals in a specific order, following the Aufbau Principle, which states that electrons fill the lowest energy orbitals first.
For example, let’s take the element oxygen (O) as an example. Oxygen is located in group 16 and period 2 of the periodic table. Based on these positions, we can determine that oxygen has 2 energy levels. To determine the electron configuration, we fill the orbitals in the following order: 1s, 2s, 2p. The first two electrons go into the 1s orbital, and the remaining 4 electrons go into the 2s and 2p orbitals, with 2 in the 2s orbital and 2 in the 2p orbital. Therefore, the electron configuration of oxygen is 1s^2 2s^2 2p^4.
In conclusion, the periodic table provides a systematic way to determine the electron configuration of an atom. By understanding the position of an element on the periodic table and following a few simple rules, we can easily determine the arrangement of electrons in an atom’s energy levels and sublevels.