Understanding the concept of polar and nonpolar molecules is essential in chemistry, as it helps determine the physical and chemical properties of substances. This worksheet provides an answer key to help students practice identifying whether a molecule is polar or nonpolar. It covers various examples and scenarios to reinforce the topic.
The key begins with a brief review of the differences between polar and nonpolar molecules. It explains how polar molecules have an uneven distribution of electron density, resulting in a positive and negative end, while nonpolar molecules have an even distribution, making them symmetrical. This information sets the foundation for the rest of the worksheet.
The answer key then presents multiple examples of molecules, asking students to identify whether they are polar or nonpolar. It includes molecules like water, carbon dioxide, methane, and more. For each example, the key provides a clear explanation of why the molecule is polar or nonpolar, using principles such as electronegativity and molecular geometry.
By using this answer key, students can check their understanding and receive immediate feedback on their practice. It allows them to self-assess their knowledge of polar and nonpolar molecules, ultimately enhancing their understanding of this important concept in chemistry.
Polar and Nonpolar Molecules Worksheet Answer Key
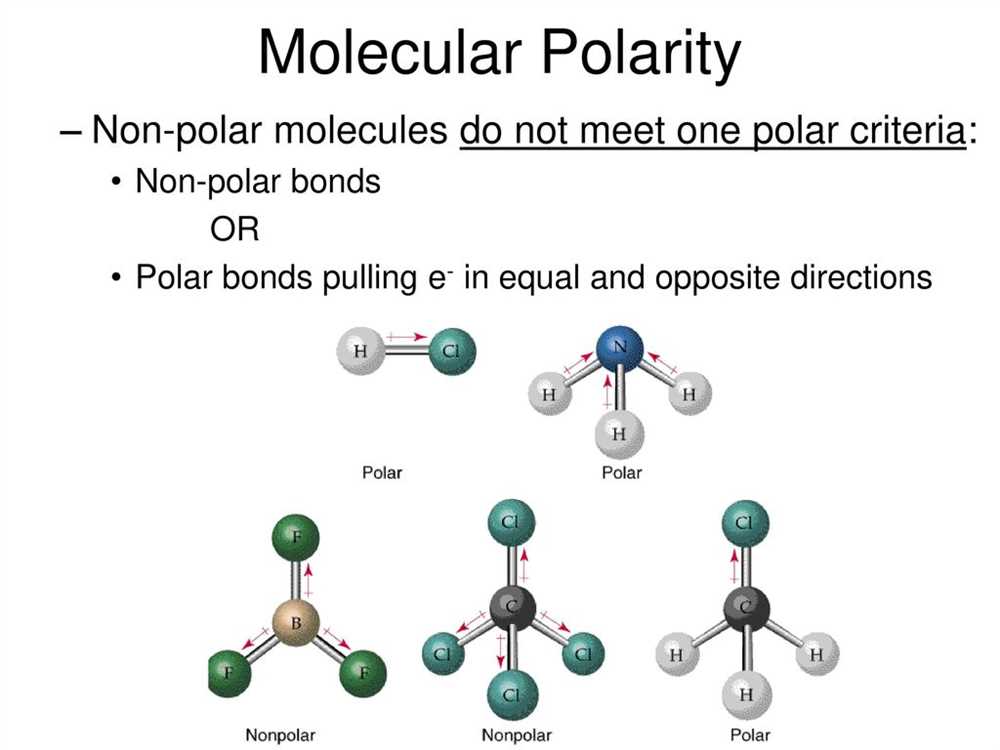
Understanding the concept of polar and nonpolar molecules is essential in the field of chemistry. In order to determine the polarity of a molecule, we look at the electronegativity difference between the atoms involved in the bond formation. If the electronegativity difference is significant, the molecule is polar; if it is close to zero, the molecule is nonpolar.
One way to identify the polarity of a molecule is to draw its Lewis structure and analyze the symmetrical arrangement of the atoms. If the molecule has a symmetrical shape, such as a tetrahedral or trigonal planar, it is likely to be nonpolar. On the other hand, if the molecule has an asymmetrical shape, such as a bent or pyramidal, it is likely to be polar.
Let’s take a look at an example from the polar and nonpolar molecules worksheet answer key. In the worksheet, we are given the molecule CH2Br2. To determine its polarity, we need to draw the Lewis structure. By doing so, we can see that the molecule has a tetrahedral shape with two chlorine atoms on one side and two hydrogen atoms on the other side, with a central carbon atom. Since the chlorine atoms are more electronegative than the hydrogen atoms, there is an uneven distribution of charge in the molecule, making it polar.
Summary:
- Polarity of a molecule is determined by the electronegativity difference between the atoms involved in the bond formation.
- A symmetrical arrangement of atoms in a molecule indicates nonpolarity, while an asymmetrical arrangement indicates polarity.
- The example molecule CH2Br2 is polar due to the uneven distribution of charge caused by the electronegativity difference between chlorine and hydrogen atoms.
What are polar and nonpolar molecules?
In chemistry, molecules can be classified as either polar or nonpolar based on their molecular structure and polarity. Polarity refers to the distribution of charges within a molecule, with polar molecules having a separation of positive and negative charges, while nonpolar molecules have an even distribution of charges.
Polar molecules occur when there is an uneven distribution of electrons within the molecule. This can happen when the atoms involved in the molecule have different electronegativities, causing a partial positive charge on one end and a partial negative charge on the other end. The polarity of a molecule is measured by its dipole moment, which indicates the strength and direction of the separation of charges. Examples of polar molecules include water (H2O), ammonia (NH3), and hydrogen chloride (HCl).
On the other hand, nonpolar molecules occur when there is an equal sharing of electrons within the molecule, resulting in an overall neutral charge. This happens when the atoms involved in the molecule have similar electronegativities or if the molecule is symmetric in shape. Nonpolar molecules do not have a dipole moment because there is no separation of charges. Examples of nonpolar molecules include oxygen gas (O2), carbon dioxide (CO2), and methane (CH4).
The polarity or nonpolarity of a molecule plays a crucial role in determining its physical and chemical properties. For example, polar molecules tend to have higher boiling and melting points, as the presence of stronger intermolecular forces makes it harder for the molecules to separate from each other. Nonpolar molecules, on the other hand, have weaker intermolecular forces and therefore lower boiling and melting points. Additionally, the polarity of a molecule affects its solubility in different solvents, as polar molecules tend to dissolve in polar solvents and nonpolar molecules dissolve in nonpolar solvents.
How do you determine if a molecule is polar or nonpolar?
In order to determine if a molecule is polar or nonpolar, you must first understand the concept of polarity in chemistry. Polarity refers to the distribution of electric charge within a molecule, which can result in the molecule having a positive and negative end, similar to a magnet. This distribution of charge can be influenced by the electronegativity of the atoms in the molecule and the molecular geometry.
One way to determine if a molecule is polar or nonpolar is to consider the molecular geometry. If the molecule has symmetry, such as being linear or having a symmetrical arrangement of atoms, it is more likely to be nonpolar. On the other hand, if the molecule has an asymmetrical arrangement of atoms, it is more likely to be polar.
Another method to determine polarity is to look at the electronegativity difference between the atoms in the molecule. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. If there is a large difference in electronegativity between the atoms in a molecule, the molecule is more likely to be polar. This is because the atom with the higher electronegativity will have a stronger pull on the shared electrons, resulting in an uneven distribution of charge.
Some examples of polar molecules include water (H2O) and ammonia (NH3). Both of these molecules have an asymmetrical arrangement of atoms, as well as a significant electronegativity difference between the atoms. This causes them to have a positive and negative end, making them polar.
On the other hand, methane (CH4) is an example of a nonpolar molecule. It has a symmetrical arrangement of atoms and no significant electronegativity difference, resulting in an even distribution of charge and a nonpolar molecule.
What is electronegativity and how does it relate to polarity?
Electronegativity is a measure of an atom’s ability to attract and hold onto electrons in a chemical bond. It is a property that varies from element to element, and is related to the position of an element in the periodic table. The higher the electronegativity of an atom, the more strongly it attracts electrons.
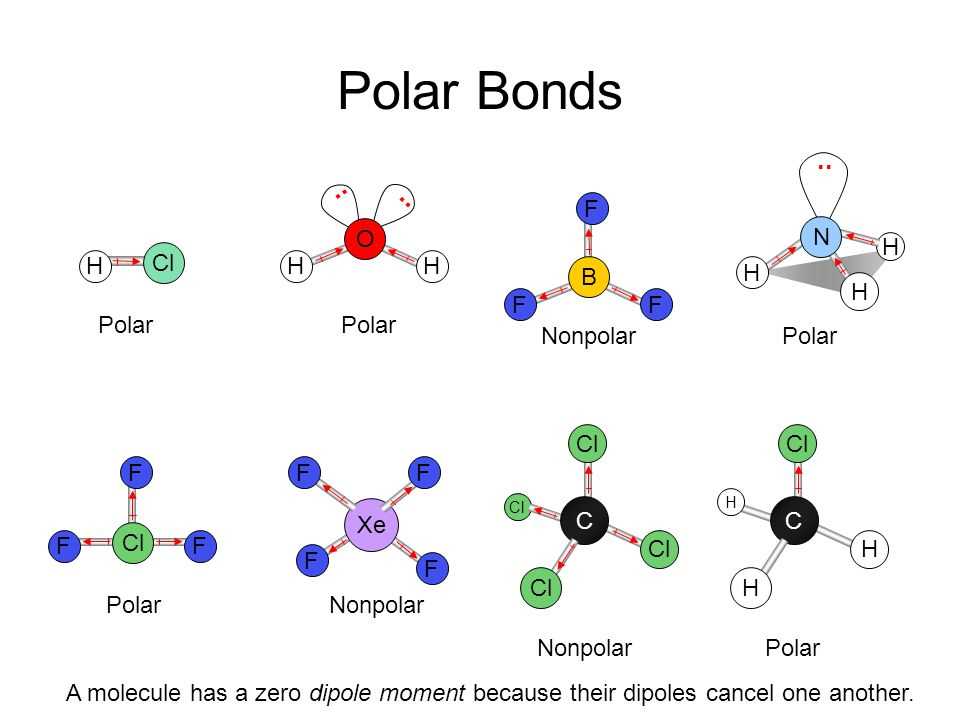
In relation to polarity, electronegativity plays a crucial role in determining whether a molecule is polar or nonpolar. When two atoms with significantly different electronegativities are bonded together, the electron pair is pulled closer to the more electronegative atom. This creates an uneven distribution of charge, with one atom becoming slightly negative (δ-) and the other becoming slightly positive (δ+), resulting in a polar bond. In contrast, in molecules with atoms of similar electronegativities, the bonding electrons are shared equally, resulting in a nonpolar bond.
In addition to individual bond polarities, the molecular shape and overall geometry also contribute to the polarity of a molecule. For example, a symmetrical molecule with polar bonds may still be nonpolar overall if the polarities cancel out due to the molecular shape. On the other hand, an asymmetrical molecule with polar bonds will typically be polar overall, as the polarities do not cancel out.
Understanding electronegativity and its relationship to polarity is important in various chemical and biological contexts. It helps explain the behavior of molecules in chemical reactions, the solubility of substances, and the interactions between molecules in biological processes such as protein folding and enzyme-substrate interactions.
Examples of Polar Molecules
In chemistry, molecules are classified into two categories: polar and nonpolar. Polar molecules are characterized by an uneven distribution of charge within the molecule. This occurs when one atom in the molecule has a stronger pull on the shared electrons than the other atom. This creates a partial positive charge on one side and a partial negative charge on the other side of the molecule.
Here are some examples of polar molecules:
- Water (H2O): Water is a polar molecule because oxygen has a stronger pull on the shared electrons than hydrogen. This results in a partial negative charge near the oxygen atom and partial positive charges near the hydrogen atoms.
- Ammonia (NH3): Ammonia is also a polar molecule. Nitrogen attracts electrons more strongly than hydrogen, causing a partial negative charge near the nitrogen atom and partial positive charges near the hydrogen atoms.
- Hydrochloric Acid (HCl): Hydrochloric acid is a polar molecule because chlorine has a higher electronegativity than hydrogen. This leads to a partial negative charge near the chlorine atom and a partial positive charge near the hydrogen atom.
- Sulfuric Acid (H2SO4): Sulfuric acid is a polar molecule due to the presence of oxygen and sulfur atoms. Oxygen attracts electrons more strongly, resulting in partial negative charges near the oxygen atoms and partial positive charges near the hydrogen atoms.
These examples demonstrate the diverse range of polar molecules found in various compounds. Understanding the polarity of molecules is crucial in predicting their behavior and interactions with other substances.
What are some examples of nonpolar molecules?

Nonpolar molecules are molecules that have an even distribution of electrons and do not have a significant positive or negative charge. In other words, the pull of electrons in the molecule is balanced, resulting in a neutral charge overall. Nonpolar molecules are typically formed by atoms that have similar electronegativities.
One example of a nonpolar molecule is carbon dioxide (CO2). Carbon dioxide consists of one carbon atom bonded to two oxygen atoms. The carbon-oxygen bonds in CO2 are polar due to the large difference in electronegativity between carbon and oxygen. However, the molecule as a whole is nonpolar because the two polar bonds are symmetrical and cancel each other out.
Another example of a nonpolar molecule is methane (CH4). Methane consists of one carbon atom bonded to four hydrogen atoms. The carbon-hydrogen bonds in methane are nonpolar because carbon and hydrogen have similar electronegativities. The molecule as a whole is nonpolar because the four polar bonds are symmetrically arranged around the carbon atom.
Other examples of nonpolar molecules include oxygen (O2), nitrogen (N2), and hydrogen (H2). These molecules consist of atoms that have similar electronegativities and do not have any polar bonds. As a result, these molecules are nonpolar and do not have any significant positive or negative charges.
Properties of Polar and Nonpolar Molecules
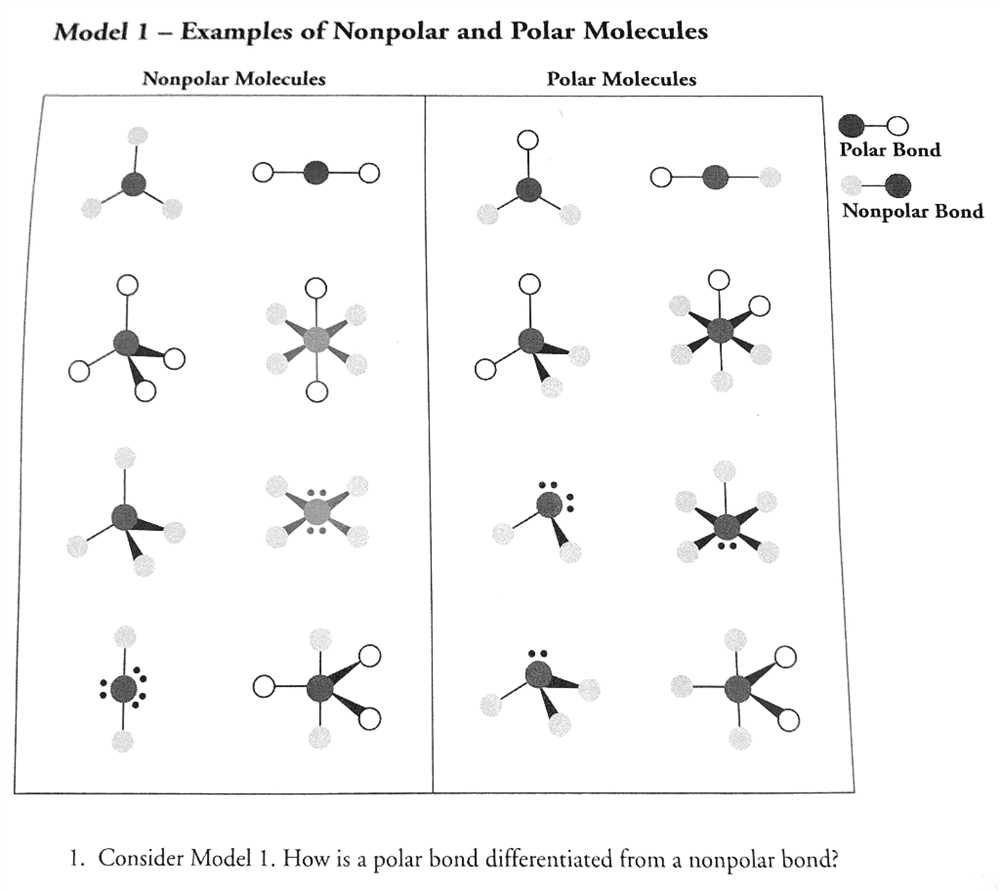
Polar and nonpolar molecules are two types of chemical compounds that differ in their physical and chemical properties. The key characteristic that distinguishes these molecules is the distribution of charge within the molecule. In a polar molecule, the distribution of electrons is uneven, resulting in a partial positive charge on one side of the molecule and a partial negative charge on the other side. Nonpolar molecules, on the other hand, have an even distribution of charge and do not have distinct positive and negative regions.
One of the main properties of polar molecules is their ability to form hydrogen bonds. Due to the partial positive and negative charges within the molecule, polar compounds can attract other polar molecules through these weak electrostatic forces. This property often leads to higher boiling and melting points for polar compounds compared to nonpolar compounds. Additionally, polar molecules tend to be soluble in polar solvents, such as water, while they are less soluble in nonpolar solvents.
Nonpolar molecules, on the other hand, do not have the ability to form hydrogen bonds or attract other polar molecules through electrostatic forces. As a result, nonpolar compounds typically have lower boiling and melting points compared to polar compounds. They are also more soluble in nonpolar solvents, such as oils and fats. Nonpolar molecules are often used as insulators or lubricants due to their low polarity and inability to dissolve in water.
In summary, the properties of polar and nonpolar molecules are determined by the distribution of charge within the molecule. Polar molecules have an uneven distribution of charge, can form hydrogen bonds, and are soluble in polar solvents. Nonpolar molecules have an even distribution of charge, cannot form hydrogen bonds, and are more soluble in nonpolar solvents. These differences in properties make polar and nonpolar molecules useful in various chemical and biological processes.
Why is it important to understand the polarity of molecules?
Polarity is a fundamental concept in chemistry that plays a crucial role in understanding the behavior and interactions of molecules. It refers to the uneven distribution of electron density within a molecule, resulting in distinct positive and negative regions. Understanding the polarity of molecules is important for a variety of reasons.
1. Predicting chemical reactivity: The polarity of a molecule can determine how it will interact with other molecules. Polar molecules tend to be more reactive and can form stronger intermolecular attractions, such as hydrogen bonds. Nonpolar molecules, on the other hand, are less likely to interact with polar molecules, limiting their reactivity. By knowing the polarity of a molecule, chemists can predict its behavior in chemical reactions and design more effective compounds.
2. Understanding solubility: The polarity of a molecule also influences its solubility in different solvents. Polar molecules are typically soluble in polar solvents, while nonpolar molecules are soluble in nonpolar solvents. This knowledge is crucial in various fields, including pharmaceuticals, where solubility plays a key role in drug delivery and formulation.
3. Determining physical properties: Polarity directly affects other physical properties of molecules, such as boiling point, melting point, and viscosity. Polar molecules tend to have higher boiling points and melting points due to the stronger intermolecular forces between their positive and negative regions. Understanding the polarity of molecules allows scientists to predict and explain these properties, which are essential in many applications, including materials science and environmental studies.
Overall, understanding the polarity of molecules is essential in many areas of science and industry. It provides valuable insights into chemical reactivity, solubility, and physical properties, ultimately leading to the development of new materials, drugs, and technologies.