If you’ve ever struggled to master the world of polyatomic ions, look no further. This comprehensive guide will provide you with an answer key to help you navigate through the complexities of polyatomic ions worksheet. Whether you’re a student studying chemistry or a teacher looking for resources to aid your students, this answer key is your ultimate tool.
In the world of chemistry, polyatomic ions are groups of atoms that are covalently bonded and carry an electric charge. They play a crucial role in understanding chemical reactions and balancing chemical equations. This worksheet is designed to test your knowledge of these ions, their names, and formulas. With the answer key, you’ll have a step-by-step breakdown of each question, making it easier to grasp even the most challenging concepts.
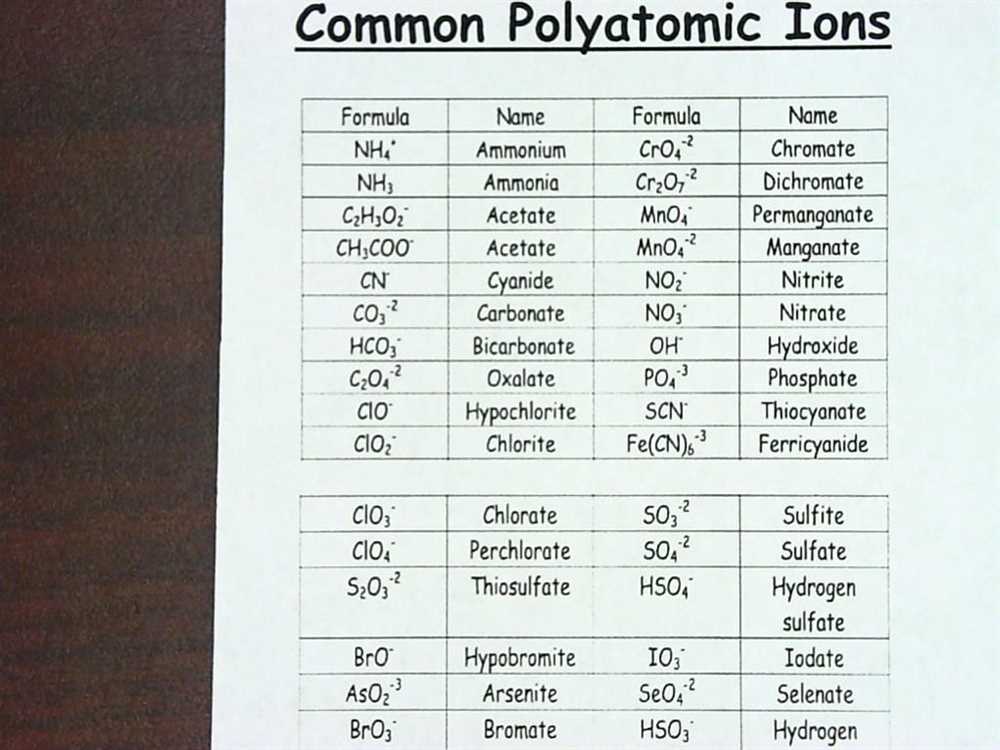
From understanding the charges and formulas of common polyatomic ions, such as sulfate (SO4^2-) and nitrate (NO3^-), to tackling more complex ions like hydroxide (OH^-) and ammonium (NH4^+), this answer key has got you covered. Each page of the worksheet is accompanied by a detailed explanation, making it an invaluable resource for both students and teachers alike.
So, whether you’re looking to ace your upcoming chemistry exam or enhance your teaching materials, the polyatomic ions worksheet answer key is your ultimate guide. By familiarizing yourself with the key concepts and practicing with the provided answers, you’ll be well-equipped to tackle any polyatomic ions question that comes your way. Dive into the world of polyatomic ions with confidence and take your understanding of chemistry to new heights!
Polyatomic Ions Worksheet Answer Key

Understanding and correctly identifying polyatomic ions is an essential skill in chemistry. A polyatomic ion is a charged particle that is made up of multiple atoms bonded together. These ions often have a specific charge and behave as a single unit in chemical reactions.
This worksheet provides practice for students to learn and memorize the names and formulas of common polyatomic ions. The answer key for this worksheet is a valuable resource that allows students to check their work and ensure they have correctly identified the ions and their charges.
The answer key includes the names and formulas of a variety of polyatomic ions, such as carbonate (CO3^2-), sulfate (SO4^2-), and nitrate (NO3^-). It also indicates the charge of each ion, which is crucial in understanding how these ions combine and react with other compounds.
By using the answer key, students can compare their answers to the correct ones and identify any mistakes or areas where they need further practice. This helps reinforce their knowledge of polyatomic ions and enhances their understanding of chemical formulas and equations.
In conclusion, the polyatomic ions worksheet answer key is an invaluable tool for students learning about polyatomic ions in chemistry. It provides a comprehensive list of ions and their charges, allowing students to practice and reinforce their knowledge. By using the answer key, students can check their work and ensure they have correctly identified polyatomic ions, helping them become proficient in this essential skill.
What are Polyatomic Ions?
Polyatomic ions are groups of atoms that are bonded together and carry an electric charge. Unlike individual atoms, which can be either positively or negatively charged, polyatomic ions are clusters of atoms that act as a single unit with a specific charge. These ions are often formed when a neutral molecule gains or loses electrons.
One common example of a polyatomic ion is the hydroxide ion, OH-. This ion consists of one oxygen atom and one hydrogen atom, and it carries a charge of negative one (-1). Another example is the ammonium ion, NH4+. This ion consists of four hydrogen atoms bonded to a central nitrogen atom, and it carries a charge of positive one (+1).
Polyatomic ions are important in chemistry because they play a crucial role in many chemical reactions. They can combine with other ions or molecules to form compounds, and their charges determine how they will interact with other substances. Understanding the properties and behavior of polyatomic ions is essential for predicting and explaining the outcomes of chemical reactions.
Understanding the Structure of Polyatomic Ions

Polyatomic ions are charged species composed of multiple atoms held together by covalent bonds. These ions have a distinct structure that determines their chemical properties and behavior. Understanding the structure of polyatomic ions is crucial for comprehending their role in chemical reactions and their overall significance in chemistry.
One key aspect of polyatomic ions is their overall electric charge, which can be positive or negative. The charge of a polyatomic ion arises from the unequal distribution of electrons within the ion. Each atom within the ion contributes towards the total charge, and this charge determines how the ion interacts with other ions and molecules. It is important to note that the overall charge of a polyatomic ion does not necessarily correspond to the charge of each individual atom within it.
The structure of polyatomic ions can be visualized as a central atom surrounded by other atoms or groups of atoms. These groups of atoms, known as ligands, are connected to the central atom through covalent bonds. The arrangement and orientation of the ligands play a crucial role in the chemical behavior of the polyatomic ion. For example, the tetrahedral structure of the ammonium ion (NH4+) allows for easy ionization and participation in various chemical reactions.
To understand the structure of polyatomic ions, it is also important to grasp the concept of resonance. Resonance occurs when there are multiple ways to arrange the bonding electrons within a polyatomic ion, leading to different possible structures. These resonance structures contribute to the overall stability and reactivity of the ion. By examining the resonance structures of polyatomic ions, chemists can better understand their unique properties and predict their behavior in different chemical reactions.
In summary, understanding the structure of polyatomic ions is essential for comprehending their chemical properties and reactivity. The overall charge, arrangement of ligands, and resonance structures contribute to the unique behavior of these ions. By studying and manipulating the structure of polyatomic ions, chemists can unlock new insights into the fascinating world of chemistry.
Important Polyatomic Ions to Know
The study of polyatomic ions is an important aspect of chemistry as it helps us understand various chemical reactions and compounds. Polyatomic ions are charged particles composed of two or more atoms that are covalently bonded and have an overall charge. Knowing the names, formulas, and charges of common polyatomic ions is crucial in predicting and balancing chemical equations.
Here is a list of some important polyatomic ions that every chemistry student should know:
- Hydroxide ion (OH-): This ion consists of an oxygen atom bonded to a hydrogen atom and carries a negative charge. It is commonly found in bases and plays a crucial role in neutralizing acids.
- Nitrate ion (NO3-): Composed of a nitrogen atom bonded to three oxygen atoms, the nitrate ion is commonly found in fertilizers. It is also involved in the nitrogen cycle and is important for plant growth.
- Ammonium ion (NH4+): Formed by combining four hydrogen atoms with a nitrogen atom, the ammonium ion is often found in compounds such as ammonium nitrate and ammonium sulfate. It is widely used in the production of fertilizers and cleaning products.
- Carbonate ion (CO3^2-): This ion is composed of a carbon atom bonded to three oxygen atoms and carries a charge of 2-. Carbonate compounds are commonly found in minerals such as limestone and play a crucial role in buffering pH in natural systems.
- Sulfate ion (SO4^2-): Consisting of a sulfur atom bonded to four oxygen atoms, the sulfate ion is widely encountered in compounds such as sulfuric acid and gypsum. It is also an important component of fertilizers.
These are just a few examples of the many polyatomic ions that exist. Understanding their formulas, charges, and roles in chemical reactions is essential for success in chemistry studies and applications.
Naming and Writing Formulas for Polyatomic Ions
When it comes to naming and writing formulas for polyatomic ions, it is important to understand the properties and characteristics of these ions. Polyatomic ions are charged molecules that consist of two or more atoms bonded together. These ions have a specific charge and can be positively or negatively charged.
One key aspect of naming polyatomic ions is recognizing their suffixes. Many polyatomic ions end in -ate or -ite. The -ate suffix indicates the ion with a higher charge or more oxygen atoms, while the -ite suffix indicates the ion with a lower charge or fewer oxygen atoms. For example, the sulfate ion (SO42-) has a higher charge and more oxygen atoms compared to the sulfite ion (SO32-).
Additionally, it is important to memorize the names and formulas of common polyatomic ions. Some examples include the carbonate ion (CO32-), the nitrate ion (NO3–), and the hydroxide ion (OH–). It is also crucial to understand the charges of these ions as they play a significant role in writing their formulas.
When writing formulas for polyatomic ions, it is important to balance the charges. The overall charge of a compound should be neutral, so it is necessary to combine the correct number of positive and negative ions to achieve this balance. For example, to write the formula for calcium carbonate (CaCO3), the charge of the calcium ion (Ca2+) and the carbonate ion (CO32-) must be balanced.
In conclusion, naming and writing formulas for polyatomic ions require an understanding of their suffixes, charges, and common names. By familiarizing oneself with these concepts and practicing, one can effectively name and write formulas for various polyatomic ions.
Practice Problems: Naming Polyatomic Ions

When working with polyatomic ions, it’s important to understand their names and charges. In this practice worksheet, you will have the opportunity to practice naming various polyatomic ions.
Here are a few examples of the problems you might encounter:
- Sulfate: Sulfate is a polyatomic ion with a charge of -2. Its chemical formula is SO4^2-. To name sulfate, you would simply state “sulfate.”
- Nitrate: Nitrate is another common polyatomic ion. It has a charge of -1 and its chemical formula is NO3^-. To name nitrate, you would say “nitrate.”
- Carbonate: Carbonate is a polyatomic ion with a charge of -2. Its chemical formula is CO3^2-. To name carbonate, you would state “carbonate.”
These examples demonstrate the naming convention for some common polyatomic ions. However, there are many more polyatomic ions to learn and practice naming. You can use this worksheet to study and practice the names and charges of various polyatomic ions.
Remember, the more you practice naming polyatomic ions, the more familiar you will become with their names and charges. This will help you in your study of chemistry and understanding the composition of different compounds. Happy practicing!
Practice Problems: Writing Formulas for Polyatomic Ions
Writing formulas for polyatomic ions is an essential skill in chemistry. Polyatomic ions are groups of atoms that are covalently bonded and carry a charge. These ions can combine with other elements or ions to form compounds. To write formulas for polyatomic ions, it is important to understand their charges and the number of each atom present in the ion.
One way to practice writing formulas for polyatomic ions is through a series of practice problems. Here are some example problems to help you improve your skills:
- Problem 1: Write the formula for the nitrate ion.
- Problem 2: Write the formula for the sulfate ion.
- Problem 3: Write the formula for the carbonate ion.
- Problem 4: Write the formula for the phosphate ion.
When approaching these problems, it is important to remember the charges and the number of atoms in each ion. The nitrate ion, for example, has a charge of -1 and consists of one nitrogen atom and three oxygen atoms. To write its formula, you would use the symbol for nitrogen (N) followed by the symbol for oxygen (O) and subscript numbers to indicate the number of each atom.
As you practice more problems, you will become more comfortable with writing formulas for polyatomic ions. Remember to review the charges and the number of atoms in each ion to ensure accuracy. Working through practice problems will help solidify your understanding and improve your ability to write formulas for polyatomic ions.
Common Mistakes to Avoid
When working with polyatomic ions, it is important to be aware of some common mistakes that are often made. By understanding these mistakes and how to avoid them, you can improve your understanding of polyatomic ions and ensure accuracy in your work.
Mistake #1: Incorrect naming conventions
One common mistake is using incorrect naming conventions when naming polyatomic ions. It is essential to remember that the names of polyatomic ions do not always follow the same patterns as binary compounds. For example, the sulfate ion (SO42-) is not called “sulfur oxide” but rather “sulfate.”
Mistake #2: Incorrect charges
Another common mistake is assigning incorrect charges to polyatomic ions. It is important to remember that the charges of polyatomic ions are fixed and cannot change. For example, the nitrate ion (NO3–) always has a charge of -1, and the ammonium ion (NH4+) always has a charge of +1. Assigning incorrect charges can lead to incorrect formulas and reactions.
Mistake #3: Ignoring parentheses
Parentheses are used to indicate the presence of multiple polyatomic ions in a compound. Ignoring or omitting parentheses can result in incorrect formulas and confusion. For example, the formula for iron (III) sulfate is Fe2(SO4)3, not FeSO4. It is important to pay attention to the presence of parentheses and use them correctly.
Mistake #4: Misidentifying polyatomic ions
Identifying and correctly naming polyatomic ions can be challenging, especially when there are several ions with similar formulas. It is important to familiarize yourself with the common polyatomic ions and their names, charges, and formulas. To avoid this mistake, it is helpful to create a study guide or reference sheet listing the commonly encountered polyatomic ions and their properties.
By being aware of these common mistakes and practicing regularly, you can improve your understanding of polyatomic ions and avoid these errors. Remember to pay attention to the naming conventions, charges, and presence of parentheses when working with polyatomic ions.