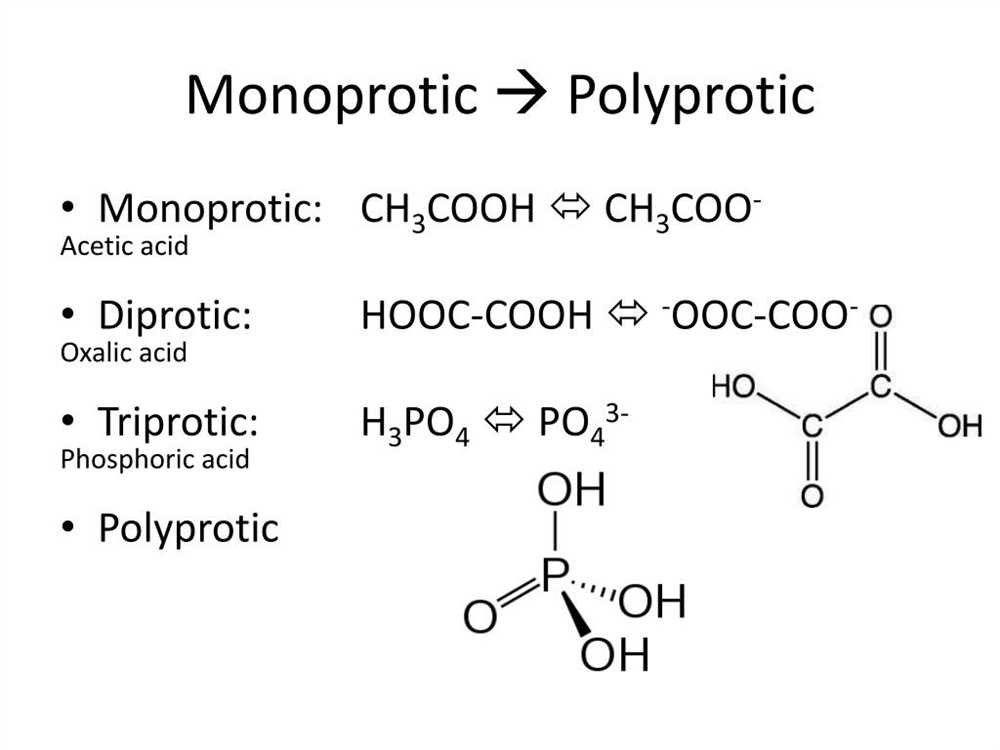
Polyprotic acids, also known as polybasic acids, are a type of acid that can donate multiple protons (H+) per molecule in a chemical reaction. These acids have unique properties and behavior compared to monoprotic acids, which can only donate one proton per molecule. Understanding the principles behind polyprotic acids is essential in various scientific fields, including chemistry and biochemistry.
One of the key characteristics of polyprotic acids is their ability to undergo stepwise ionization, meaning that they donate their protons in a sequential manner rather than all at once. This process occurs in stages, with each stage resulting in the formation of a different conjugate base. As a result, polyprotic acids have multiple dissociation constants (Ka values) corresponding to each of these stages.
The behavior of polyprotic acids can be represented using acid-base equilibrium equations. These equations help determine the concentration of different species in a solution, such as the acid, its conjugate base, and the hydronium ion. By solving these equations, scientists can calculate the pH of a polyprotic acid solution and determine its strength and acidity.
Understanding the properties of polyprotic acids is crucial in various scientific applications. For example, in the field of biochemistry, it is essential to understand how polyprotic acids, such as amino acids, behave in different physiological environments. Additionally, in chemistry, knowledge of polyprotic acids helps in the design and optimization of chemical reactions involving acids and bases.
In conclusion, polyprotic acids are a unique class of acids that can donate multiple protons. Their stepwise ionization and multiple dissociation constants make them distinct from monoprotic acids. Understanding the properties and behavior of polyprotic acids is essential in various scientific fields, allowing for a better understanding of acid-base reactions and their applications.
Polyprotic Acids POGIL Answer Key
Polyprotic acids are acids that contain more than one ionizable hydrogen atom. They can donate multiple protons (H+) in a series of ionization reactions. Understanding the behavior of polyprotic acids is essential in chemistry, as it helps predict their chemical properties, acidity, and reactivity.
The Polyprotic Acids POGIL (Process Oriented Guided Inquiry Learning) activity provides students with a structured approach to explore and understand the concept of polyprotic acids. The activity involves a series of guided questions and data analysis to help students develop a deeper understanding of the topic.
Here is an example of the key concepts covered in the Polyprotic Acids POGIL activity:
- Ionization reactions: Students learn how polyprotic acids undergo multiple ionization reactions, in which hydrogen ions are sequentially removed from the molecules.
- Equilibrium constants: The activity provides an opportunity to calculate the equilibrium constants for each ionization reaction and determine the acidity of the resulting solutions.
- Buffer solutions: Students explore how polyprotic acids can act as buffer solutions, regulating the pH of a solution by resisting changes in acidity.
- Titration curves: The POGIL activity includes the analysis of titration curves for polyprotic acids, helping students understand the pH changes during a titration process.
This Polyprotic Acids POGIL Answer Key serves as a resource for students to check their understanding and verify their answers. It provides step-by-step explanations and solutions to the questions posed in the activity, ensuring that students grasp the important concepts and principles of polyprotic acids.
By engaging in the Polyprotic Acids POGIL activity and using the answer key as a reference, students can enhance their understanding of polyprotic acids and develop their problem-solving skills in the field of chemistry. This guided inquiry approach empowers students to actively participate in the learning process and apply their knowledge to real-world scenarios.
Overview of Polyprotic Acids
Polyprotic acids are compounds that can donate more than one proton (H+) in an aqueous solution. These acids contain multiple ionizable hydrogen atoms, which can be sequentially ionized to form multiple dissociation steps. Each dissociation step is characterized by its own equilibrium constant, and the overall acidity of the polyprotic acid depends on the strengths of these individual ionization reactions.
One example of a polyprotic acid is phosphoric acid (H3PO4), which can donate up to three protons. The first dissociation step produces the monoprotic acid H2PO4-, the second step yields the diprotic acid HPO42-, and the final step results in the formation of the triprotic acid PO43-. The acid dissociation constants (Ka) for these three steps are different, with the first step being the strongest and the third step being the weakest.
Understanding the behavior of polyprotic acids is important in various areas, including chemistry, biochemistry, and environmental science. It allows scientists to predict the pH of solutions containing these acids, as well as their reactivity with other substances. The concept of acid-base equilibria and ionization constants plays a crucial role in determining the overall behavior of polyprotic acids.
The behavior of polyprotic acids can be analyzed using mathematical equations and calculations. These calculations involve determining the concentrations of different species in solution at various equilibrium points, as well as determining the degree of ionization for each dissociation step. Furthermore, the use of titration techniques can help determine the equivalence points and the corresponding pH values for the different ionization steps.
Overall, studying polyprotic acids allows scientists to gain a deeper understanding of acid-base chemistry and provides valuable insights into the behavior of complex chemical systems. By analyzing the individual ionization steps and their equilibrium constants, researchers can make predictions about the behavior of polyprotic acids in various chemical and biological processes.
Structure and Properties of Polyprotic Acids
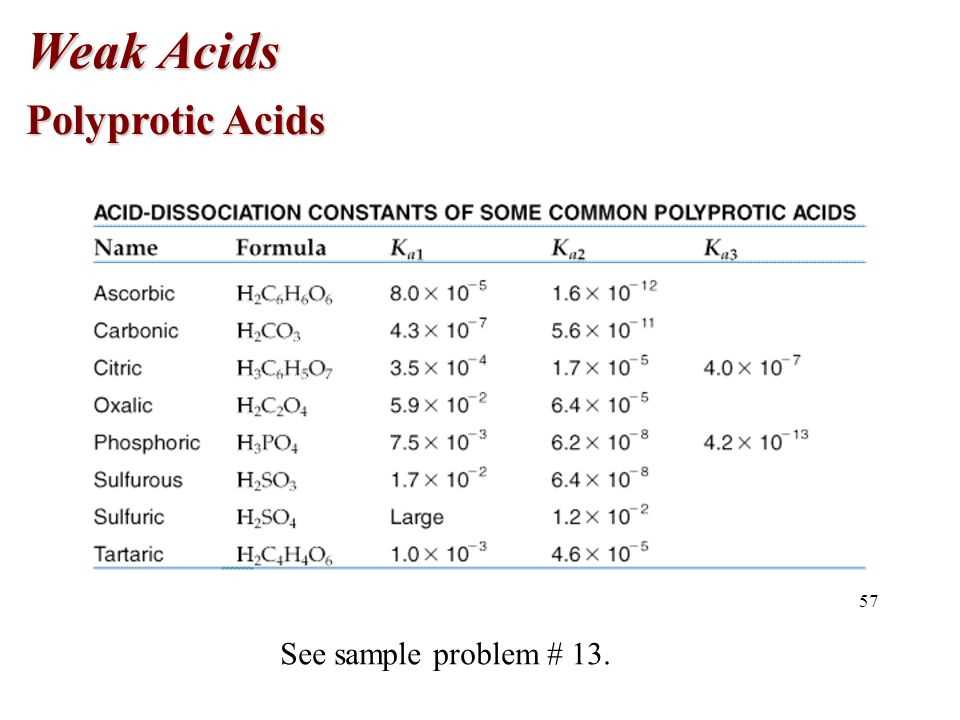
Polyprotic acids are acids that contain more than one ionizable hydrogen atom. They can donate multiple protons to water or other bases. The structure and properties of polyprotic acids can be understood by examining their molecular structure and the acidity constants associated with each ionizable hydrogen atom.
The molecular structure of a polyprotic acid determines its ability to donate protons. Each ionizable hydrogen atom is bonded to an oxygen atom, and the strength of this bond determines the acidity of the molecule. The more electronegative the atoms surrounding the hydrogen atom are, the weaker the bond will be, and the more easily the hydrogen atom can be dissociated. Therefore, polyprotic acids with more electronegative elements, such as oxygen or nitrogen, will be more acidic.
The acidity constants, or dissociation constants, of polyprotic acids describe the degree to which each ionizable hydrogen atom dissociates in water. These constants, denoted as Ka1, Ka2, Ka3, etc., represent the equilibrium constant for the dissociation of a proton from the first, second, third, etc. ionizable hydrogen atom. The larger the value of Ka, the stronger the acid. Additionally, the acidity constants follow a decreasing trend, as each subsequent ionizable hydrogen atom is less acidic than the previous one.
In summary, the structure and properties of polyprotic acids are determined by the molecular structure and acidity constants associated with each ionizable hydrogen atom. Understanding these factors can help predict the behavior of polyprotic acids in various chemical reactions and solutions.
Acid-Base Equilibria and Ionization Constants
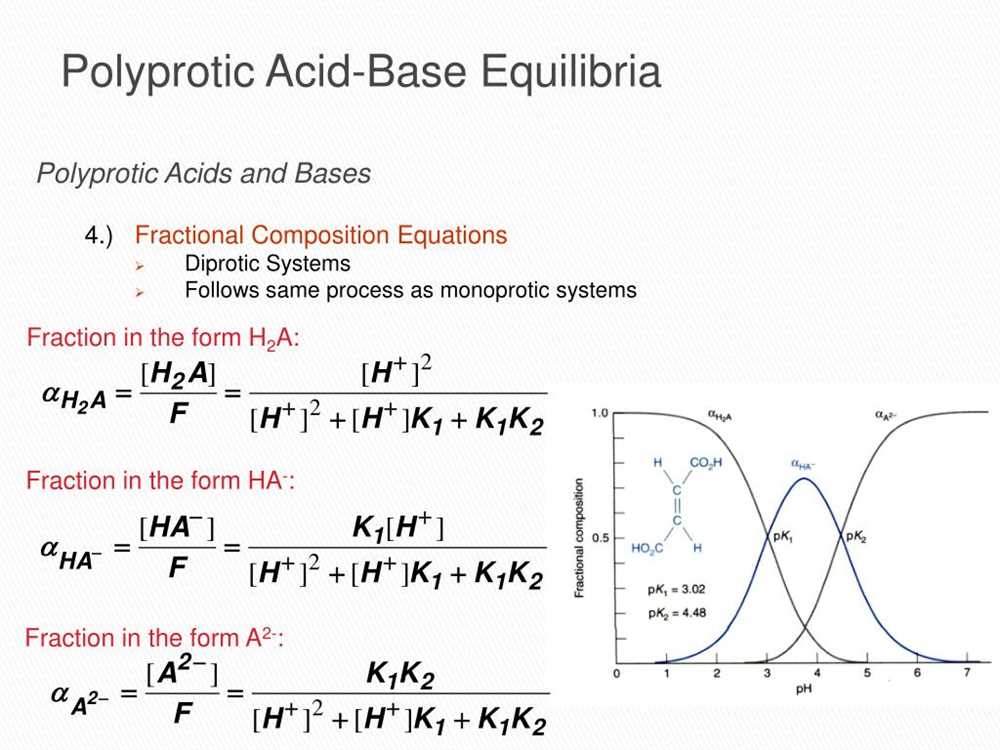
Acid-base equilibria are fundamental to understanding the behavior of polyprotic acids and their ionization constants. Polyprotic acids are compounds that contain multiple ionizable hydrogen atoms. When these acids are dissolved in water, they undergo stepwise ionization reactions, where each ionizable hydrogen atom is sequentially removed. The ionization constants, also known as acid dissociation constants (Ka), describe the extent to which each hydrogen atom dissociates from the acid.
Initially, the first hydrogen atom dissociates from the acid, forming the first conjugate base. The strength of this acid is described by the value of Ka1. As the acid continues to lose hydrogen atoms, subsequent ionization reactions occur, resulting in the formation of additional conjugate bases and acid dissociation constants (Ka2, Ka3, etc.). These ionization constants vary depending on the specific acid and the stability of the conjugate bases.
Understanding polyprotic acids and their ionization constants is crucial in various areas of chemistry. It allows us to predict acid-base reactions, calculate pH values, and determine the concentrations of acidic or basic species in a solution. Additionally, knowing the ionization constants helps in identifying the strengths and weaknesses of different acids and bases, contributing to the development of pharmaceuticals, environmental studies, and chemical analysis.
In conclusion, acid-base equilibria and ionization constants play a significant role in understanding the behavior of polyprotic acids. The stepwise ionization reactions and the determination of ionization constants allow for predictions and calculations related to acid-base chemistry, pH, and concentration of species in solution. This knowledge is essential for various applications in different scientific fields.
Ionization Constants and pH
Ionization constants play a crucial role in determining the pH of a solution. When a polyprotic acid dissolves in water, it ionizes to release hydrogen ions (H+). These ions can affect the acidity of the solution and determine its pH. The ionization constant, also known as the acid dissociation constant (Ka), quantifies the extent of ionization of a polyprotic acid in water.
The ionization constant can be calculated using the concentration of the dissociated species and the undissociated species. The higher the value of Ka, the stronger the acid, indicating a greater extent of ionization. A low value of Ka indicates a weak acid with lesser ionization. pH, on the other hand, measures the acidity or alkalinity of a solution and is calculated using the negative logarithm of the hydrogen ion concentration. pH can range from 0 to 14, with 7 being considered neutral.
The presence of multiple ionization constants in polyprotic acids makes the calculation of pH more complex. Each ionization constant corresponds to a specific step in the ionization process. As a result, the pH of a polyprotic acid solution may vary depending on which constant is used for the calculation. It is important to consider all the ionization steps and their corresponding ionization constants to accurately determine the pH of a polyprotic acid solution.
In conclusion, ionization constants provide valuable information about the extent of ionization of a polyprotic acid in water. These constants, along with the concentration of dissociated and undissociated species, can be used to calculate the pH of a solution. Understanding the concept of ionization constants and their role in pH calculations is essential in analyzing the acidity or alkalinity of polyprotic acid solutions.
Acid Strength and Ionization Trends
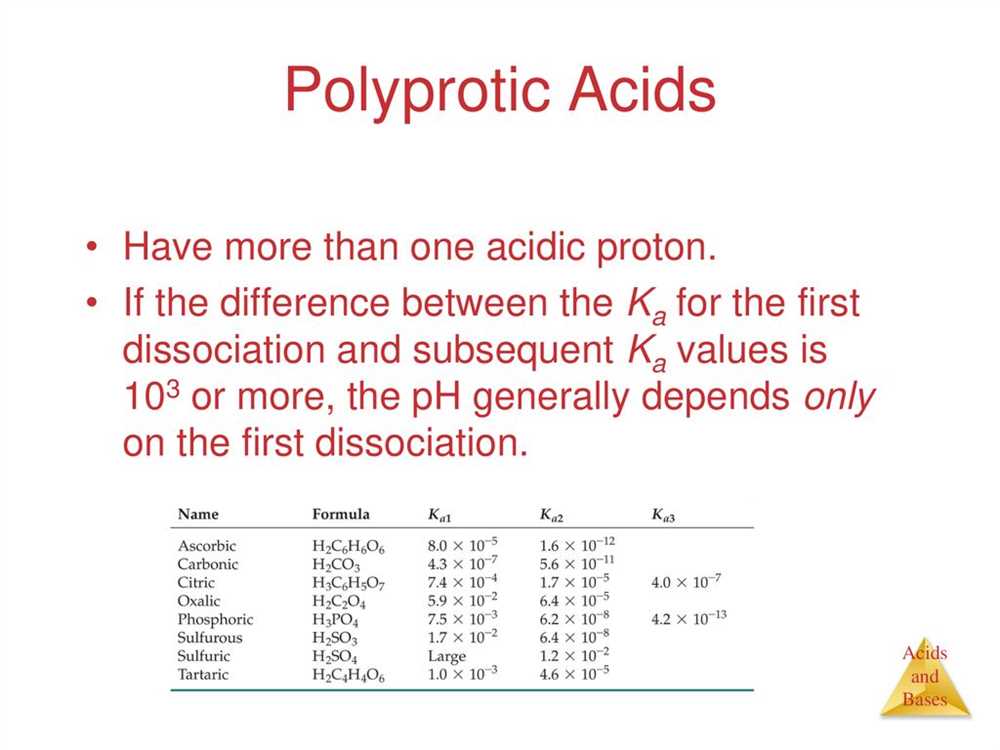
Acid strength is a fundamental concept in chemistry that determines the ability of an acid to donate protons (H+) in a chemical reaction. It is influenced by several factors, including the acid’s molecular structure and the presence of any electron withdrawing or donating groups. By understanding the trends in acid strength, scientists can predict and explain various chemical reactions.
One key trend in acid strength is the relationship between the acidity of an acid and its dissociation constant (Ka). The dissociation constant is a measure of the extent to which an acid ionizes in water. The higher the value of Ka, the stronger the acid. For example, hydrochloric acid (HCl) has a high Ka value, indicating that it completely ionizes in water and is a strong acid. On the other hand, acetic acid (CH3COOH) has a low Ka value, indicating that it only partially ionizes and is a weak acid.
Another trend in acid strength is the effect of electronegativity on acidity. Electronegativity is a measure of an atom’s ability to attract electrons towards itself in a chemical bond. Generally, acids with highly electronegative atoms, such as chlorine or fluorine, tend to be stronger than those with less electronegative atoms. This is because the electronegative atom pulls the electrons towards itself, making it easier for the acid to donate a proton.
The presence of electron withdrawing or donating groups in an acid can also influence its acidity. Electron withdrawing groups, such as nitro (-NO2) or carbonyl (C=O) groups, tend to increase the acidity of an acid by destabilizing the conjugate base. On the other hand, electron donating groups, such as alkyl (R) groups, tend to decrease acidity by stabilizing the conjugate base. This can be seen in the comparison of chloroacetic acid (ClCH2COOH) and acetic acid (CH3COOH). The presence of the electron withdrawing chlorine atom in chloroacetic acid increases its acidity compared to the electron donating methyl group in acetic acid.
In conclusion, acid strength is influenced by several factors, including the dissociation constant, electronegativity, and the presence of electron withdrawing or donating groups. Understanding these trends is essential for predicting and explaining the behavior of acids in chemical reactions.
Titration Curves and Acid-Base Titrations
An acid-base titration is a laboratory technique used to determine the concentration of an unknown acid or base solution. By adding a known volume of a standard solution of acid or base to the unknown solution in a controlled manner, the point at which the reaction between the acid and base is complete can be determined. This point is called the equivalence point. The titration curve is a graphical representation of the pH of the solution being titrated as a function of the volume of added acid or base.
Titration curves are typically S-shaped due to the buffering capacity of the solution. At the beginning of the titration, the pH remains relatively constant as the added acid or base is neutralized by the buffer. As the titration continues, the pH starts to change more rapidly until it reaches an inflection point, which corresponds to the halfway point of the reaction. This is known as the half-equivalence point. After the half-equivalence point, the pH again becomes relatively constant until the equivalence point is reached.
Key features of a titration curve:
- Initial pH: The pH before any acid or base is added.
- Highest pH: The pH at the equivalence point, where the acid and base have reacted completely.
- Half-equivalence point: The point at which half of the acid or base has been neutralized.
- Buffer region: The region where the pH remains relatively constant due to the presence of a buffer.
Titration curves provide valuable information about the nature of the acid or base being titrated. The shape of the curve can indicate whether the acid is monoprotic, diprotic, or polyprotic. Additionally, the equivalence point can be used to calculate the concentration of the unknown solution.
| Acid | Equivalence Point pH |
|---|---|
| Monoprotic acid | 7.00 |
| Diprotic acid | 5.00 and 9.00 |
| Polyprotic acid | Varies depending on the number of ionizable hydrogen atoms |
In conclusion, titration curves and acid-base titrations provide a quantitative method for determining the concentration of an unknown acid or base solution. By analyzing the pH changes throughout the titration, important information about the nature of the acid or base can be obtained. Understanding titration curves and their interpretation is essential in analytical chemistry and other related fields.