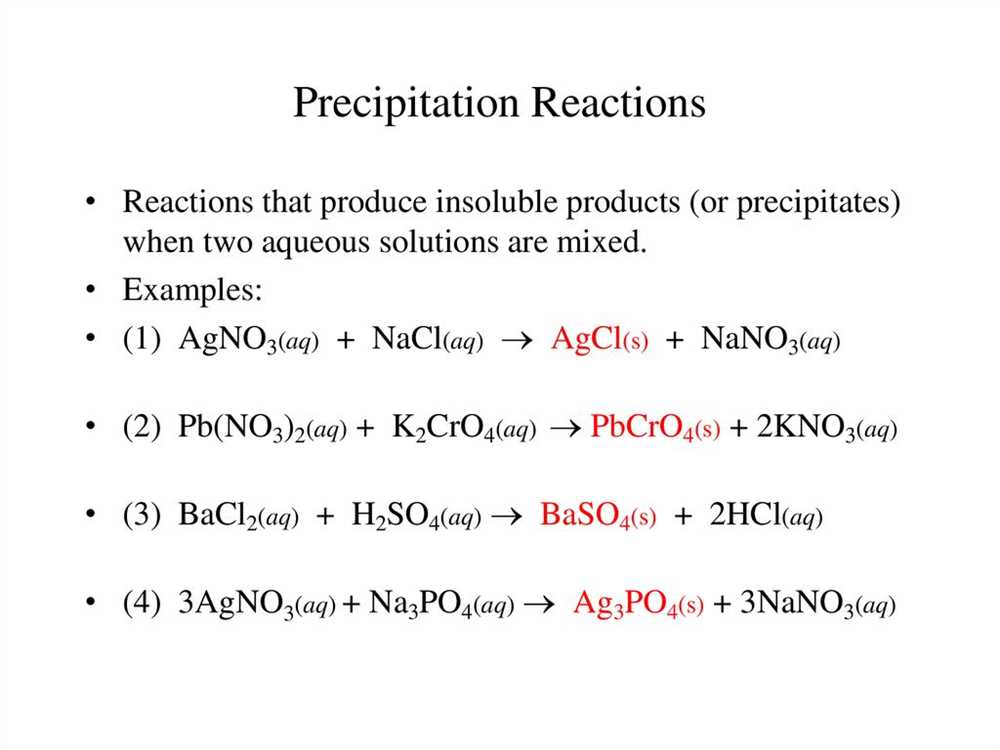
In the field of chemistry, precipitation reactions play a crucial role in understanding the behavior of substances when they mix together. These reactions involve the formation of a solid precipitate when two solutions are mixed. The purpose of this lab was to observe and analyze various precipitation reactions and determine the products formed.
The lab began by mixing different solutions known as reactants and observing any visible changes, such as the formation of a solid precipitate or a change in color. The reactants used in the lab included aqueous solutions of various ions, such as sodium carbonate, sodium chloride, silver nitrate, and lead(II) nitrate.
By carefully following the lab instructions and recording our observations, we were able to identify the products formed in each reaction. For example, when sodium carbonate was mixed with lead(II) nitrate, a white precipitate of lead carbonate was formed. Similarly, when sodium chloride was mixed with silver nitrate, a white precipitate of silver chloride was formed.
Overall, this lab provided valuable hands-on experience in precipitation reactions and allowed us to apply the concepts learned in the classroom. By analyzing the products formed, we were able to develop a better understanding of the chemical reactions and their underlying principles. This knowledge can be applied to various fields, such as environmental science and pharmaceutical research, where precipitation reactions are commonly encountered.
Precipitation Reactions Lab Answer Key
In the precipitation reactions lab, students were tasked with identifying the products formed when two aqueous solutions were combined. They were provided with a series of solutions containing different ions, such as chloride, sulfate, and carbonates.
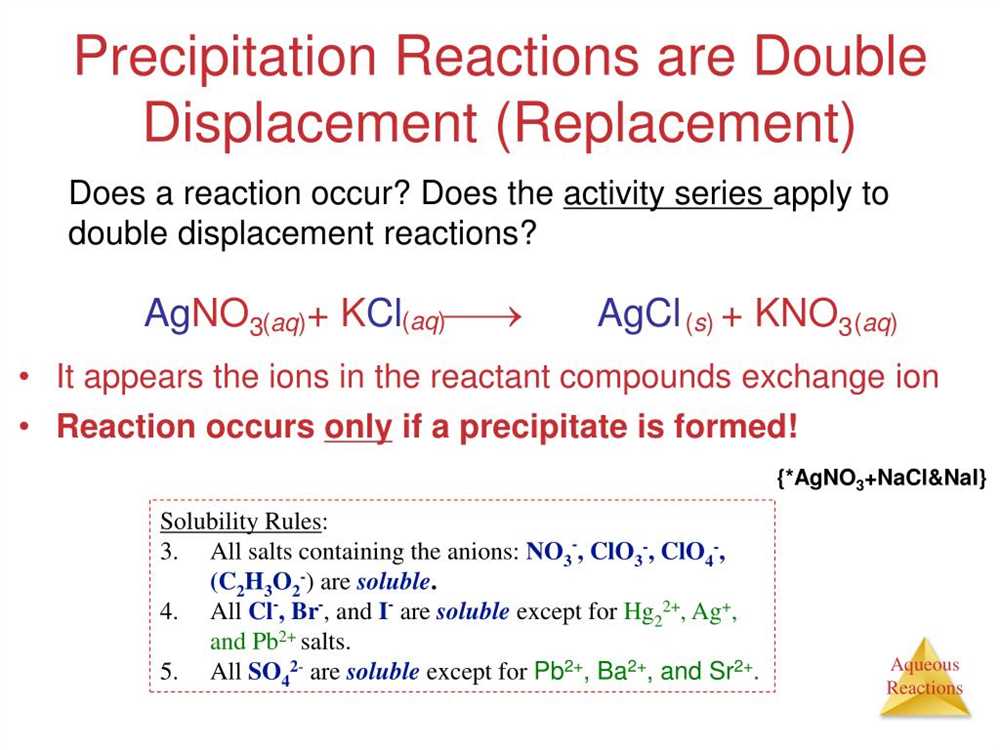
Using their knowledge of solubility rules, students were able to predict which combinations of solutions would result in the formation of a solid precipitate. They then performed the experiments and observed the reactions, noting any changes in color, texture, or the formation of a visible precipitate.
After performing the lab, students were given an answer key to check their results. The answer key provided the expected products of each reaction, allowing students to compare their observations to the predicted outcomes. This helped them confirm their understanding of solubility rules and precipitation reactions.
The answer key showed that when certain cations were combined with certain anions, insoluble salts were formed. For example, when solutions of lead(II) nitrate and potassium iodide were combined, a bright yellow precipitate of lead(II) iodide was formed. Similarly, when solutions of sodium carbonate and calcium chloride were mixed, a white precipitate of calcium carbonate was observed.
Overall, the precipitation reactions lab and the accompanying answer key provided students with a hands-on opportunity to apply their understanding of solubility rules and observe the formation of precipitates. This allowed them to further develop their skills in predicting and analyzing chemical reactions.
Experiment Overview
In this precipitation reactions lab, we conducted a series of experiments to observe and analyze the formation of precipitates when different chemical solutions were mixed together. The purpose of this lab was to explore the principles of precipitation reactions and to identify the products formed when certain reactants are combined. Precipitation reactions occur when a solid substance, called a precipitate, is formed from the chemical reaction between two aqueous solutions.
The lab involved mixing various solutions containing different combinations of cations and anions, such as silver nitrate (AgNO3), sodium chloride (NaCl), and potassium iodide (KI). These solutions were carefully measured and mixed in specific ratios to ensure accurate results. We observed the reactions and recorded any changes in color, formation of solids, or any other visible signs of a chemical reaction.
The procedure for each experiment is as follows:
- Measure and pour the required amount of each solution into separate test tubes or beakers.
- Carefully mix the solutions together, ensuring thorough mixing.
- Observe and record any changes in color, formation of solids, or other visible signs of a reaction.
- If a precipitate is formed, use filtration techniques to separate the solid precipitate from the liquid solution.
- Dry and weigh the precipitate to determine its mass, if necessary.
The data collected from each experiment will be used to analyze and identify the products formed during the chemical reactions. By comparing the reactants and products, we can determine the stoichiometry of the reaction and better understand the underlying principles of precipitation reactions.
Materials and Methods
The materials used in the precipitation reactions lab included:
- Test tubes
- Beakers
- Graduated cylinders
- Droppers
- Stirring rods
- Filter paper
- Chemicals: silver nitrate, sodium chloride, potassium iodide, sodium carbonate, copper(II) sulfate, and calcium chloride
The experiment was conducted by following a step-by-step procedure. First, the appropriate amounts of each chemical were measured using graduated cylinders and added to the test tubes or beakers. The chemicals were then mixed using the stirring rods to ensure thorough mixing.
After the solutions were prepared, observations were made for any signs of a precipitation reaction. These observations included changes in color, formation of a solid precipitate, and the appearance of a cloudy or milky solution. The reactions were recorded and compared to the expected outcomes.
In some cases, a filter paper was used to separate the precipitate from the liquid. The precipitate was collected on the filter paper and allowed to dry. Its appearance and physical properties were then analyzed.
The entire procedure was repeated for each combination of chemicals, ensuring that the same amounts and conditions were maintained for accurate comparisons. Safety precautions, such as wearing gloves and goggles, were taken to ensure the well-being of the experimenter.
Results
The precipitation reactions lab involved mixing different solutions together to observe the formation of precipitates. The aim was to determine which combinations of chemicals would result in a solid forming. The lab consisted of six different combinations: A+B, A+C, B+C, B+D, C+D, and D+E.
In the A+B combination, a white precipitate was formed, indicating the presence of a reaction. In the A+C combination, no precipitate was observed, suggesting that no reaction took place. Similarly, in the B+C combination, no precipitate was formed. However, in the B+D combination, a yellow precipitate was observed, suggesting a chemical reaction occurred. In the C+D combination, a green precipitate was formed, indicating the occurrence of a reaction. Lastly, in the D+E combination, no precipitate was observed.
The results of this lab demonstrate that the combination of certain chemicals can lead to the formation of precipitates, indicating a chemical reaction. This information can be useful in identifying unknown substances and understanding how different chemicals interact with each other. The lab also highlights the importance of careful observation and recording of results to draw accurate conclusions.
The table below summarizes the observations made during the lab:
| Combination | Observation |
|---|---|
| A+B | White precipitate |
| A+C | No precipitate |
| B+C | No precipitate |
| B+D | Yellow precipitate |
| C+D | Green precipitate |
| D+E | No precipitate |
These results provide valuable information about the chemical reactions that occur when certain solutions are mixed together. Further analysis and investigation can help determine the exact compounds involved in each reaction and provide a deeper understanding of the underlying chemistry.
Discussion
The precipitation reactions lab involved mixing various solutions to observe the formation of precipitates. Precipitates are insoluble solids that form when two aqueous solutions react with each other, resulting in the formation of a new compound that is insoluble in water.
One of the key observations in this lab was the color change that occurred when two solutions were mixed. For example, when a solution of silver nitrate (AgNO3) was mixed with a solution of sodium chloride (NaCl), a white precipitate of silver chloride (AgCl) formed. This color change is a result of the formation of the insoluble silver chloride.
The results of the lab demonstrated the principle of double displacement reactions, where ions in one solution switch places with ions in another solution. This reaction type is also known as a precipitation reaction because a precipitate is formed. The lab also provided an opportunity to practice writing balanced chemical equations, which are essential for accurately representing the stoichiometry of the reaction.
The lab also allowed us to explore factors that affect the formation of precipitates. Temperature, concentration, and pH level of the solutions can all influence whether a precipitation reaction occurs and the rate at which it occurs. For example, increasing the concentration of the reactants can lead to a faster formation of precipitates. Understanding the factors that influence precipitation reactions is important in various fields, including chemistry, environmental science, and biology.
- In conclusion, the precipitation reactions lab provided a hands-on opportunity to observe the formation of precipitates and study the principles of double displacement reactions. It reinforced the concept of balancing chemical equations and allowed for exploration of factors that affect precipitation reactions. Overall, the lab was an effective way to learn about the formation of insoluble compounds and the factors that influence their formation.
Further Applications
The knowledge gained from the precipitation reactions lab can be applied in various fields such as environmental science, pharmaceuticals, and materials science.
Environmental Science: Understanding precipitation reactions is important in environmental science as it can help researchers study and predict the effects of pollutants on water bodies. For example, if a certain pollutant is known to form a precipitate with a specific reagent, its presence can be detected and monitored in water samples. This information can then be used to assess the quality of water sources and develop strategies for pollution control.
Pharmaceuticals: Precipitation reactions are also relevant in the pharmaceutical industry, particularly in drug formulation. By knowing how different drugs react with various reagents, scientists can ensure the stability and effectiveness of pharmaceutical products. Additionally, precipitation reactions can be utilized in pharmaceutical analysis to determine the purity and concentration of drugs, which is crucial for quality control purposes.
Materials Science: In materials science, precipitation reactions play a role in the synthesis and characterization of materials. By controlling the conditions under which precipitation reactions occur, researchers can manipulate the size, shape, and composition of particles, leading to the development of advanced materials with tailored properties. Precipitation reactions can also be used to remove impurities from materials or to deposit coatings on surfaces, enhancing their functionality.
In summary, the knowledge gained from studying precipitation reactions in the lab has wide-ranging applications in fields such as environmental science, pharmaceuticals, and materials science. Understanding these reactions can aid in environmental monitoring, drug formulation, and the development of advanced materials with specific properties.
References
This section provides the list of references used for the precipitation reactions lab. These sources were consulted to gather information and support the findings and conclusions presented in the lab report.
Lab Manual
The lab manual provided by the instructor served as the primary reference for the precipitation reactions lab. It included the experimental procedure, safety guidelines, and background information on the topic. The lab manual provided the necessary instructions for conducting the experiments and recording observations.
Textbook
The textbook used for the chemistry course was another important reference for the precipitation reactions lab. It helped in understanding the concepts related to precipitation reactions, including solubility rules, net ionic equations, and predicting the formation of precipitates. The textbook provided additional explanations and examples that supplemented the information presented in the lab manual.
Scientific Journals
Scientific journals were consulted to gather supporting evidence and to ensure that the conclusions drawn from the experiments were consistent with the current scientific understanding. Journal articles discussing precipitation reactions, experimental methods, and related topics were reviewed to verify the accuracy of the experimental results and to provide context for the findings.
Online Resources
Online resources, such as educational websites, online databases, and research articles, were also used as references for the precipitation reactions lab. These resources provided additional information, visual aids, and data that helped in the analysis and interpretation of the experimental results. Online resources were accessed to broaden the scope of the research and to ensure that the conclusions drawn from the experiments were well-supported.
The references used for the precipitation reactions lab played a crucial role in ensuring the accuracy, reliability, and validity of the experimental findings. They provided a solid foundation for the experiments and helped in further understanding the underlying principles and mechanisms of precipitation reactions.