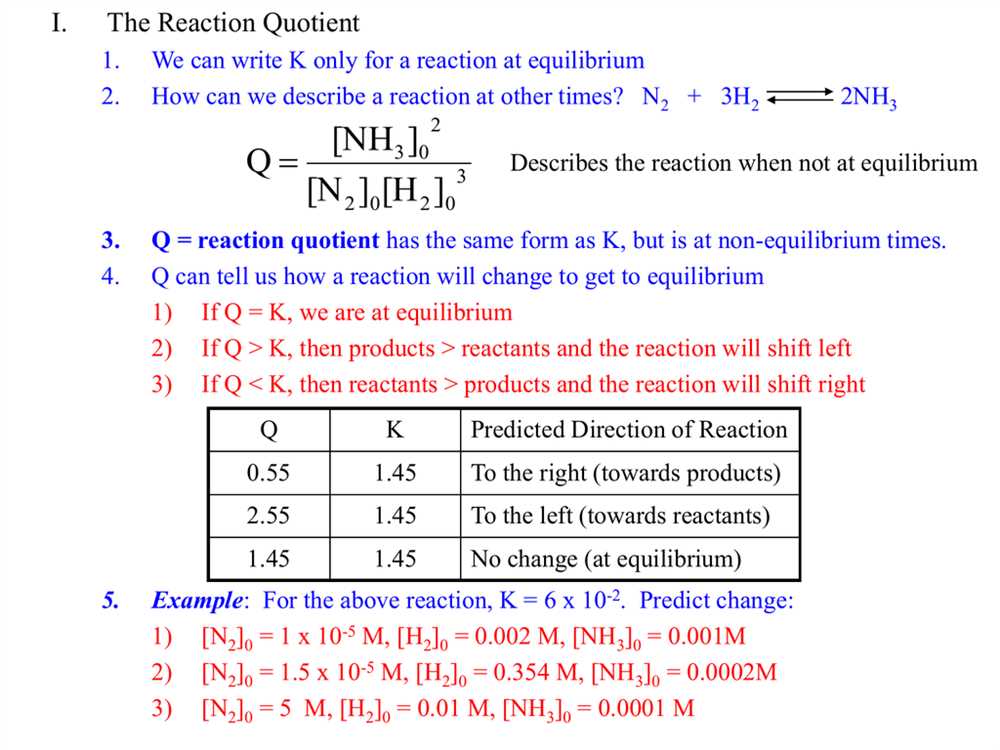
In chemistry, the rate of reaction refers to how quickly a reaction takes place. Understanding the factors that affect the rate of a reaction is crucial in predicting and controlling chemical reactions. One of the methods used to analyze these factors is the POGIL (Process Oriented Guided Inquiry Learning) approach.
POGIL activities provide students with a structured framework to explore and analyze chemical reactions. Students are given a set of guided questions and activities that encourage them to think critically and actively engage with the subject matter. These activities not only enhance students’ understanding of the concepts but also promote collaborative learning and problem-solving skills.
When it comes to rate of reaction, POGIL activities delve into topics such as concentration, temperature, and catalysts. By analyzing experimental data and performing calculations, students can determine the impact of these factors on the rate of a reaction. Additionally, POGIL activities help students develop a deeper understanding of the concept of reaction rate by connecting it to real-life examples and applications.
Overall, POGIL activities provide a valuable tool for students to explore and comprehend the factors that influence the rate of a reaction. By actively engaging in this learning approach, students can deepen their understanding of chemical reactions and develop the critical thinking skills necessary for success in the field of chemistry.
What is the rate of reaction?
The rate of reaction refers to how quickly a chemical reaction takes place. It is defined as the change in concentration of a reactant or product per unit of time. In other words, it measures how fast the reactants are converted into products or how fast the products are formed.
The rate of reaction can be influenced by various factors, such as temperature, concentration, pressure, and the presence of catalysts. Increasing the temperature generally increases the rate of reaction, as it provides more energy for the particles to collide and react. Similarly, increasing the concentration of reactants or the pressure can lead to more frequent collisions and a higher rate of reaction. Catalysts, on the other hand, can lower the activation energy required for a reaction to occur, thus speeding up the rate of reaction.
The rate of reaction is typically expressed as the change in concentration of a reactant or product over a specific time period. It can be determined experimentally by measuring the concentration of a substance at different time points during a reaction. The rate can be calculated using the formula:
Rate of reaction = (Change in concentration of reactants or products) / (Change in time)
In summary, the rate of reaction measures how quickly a chemical reaction occurs and can be influenced by various factors. Understanding the rate of reaction is important in fields such as chemistry and chemical engineering, as it allows scientists and engineers to optimize reaction conditions, predict reaction outcomes, and design efficient chemical processes.
Factors affecting the rate of reaction
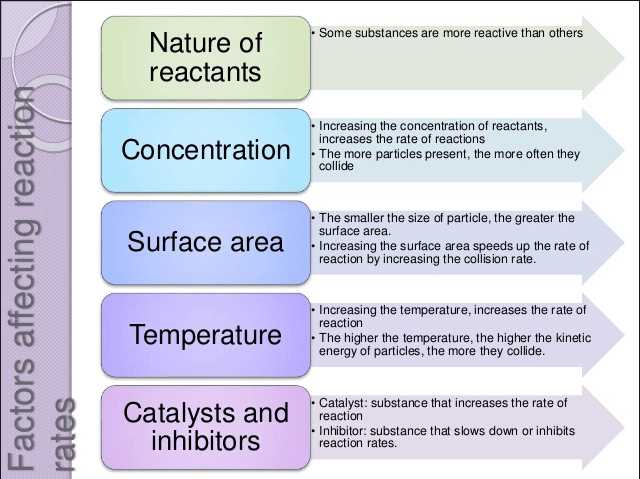
The rate of a chemical reaction is influenced by several factors that affect the speed at which reactants are converted into products. These factors include the concentration of reactants, temperature, catalysts, and surface area.
Concentration of reactants: The rate of a reaction is directly proportional to the concentration of reactants. As the concentration of reactants increases, the number of collisions between particles also increases, leading to more successful collisions and a faster rate of reaction.
Temperature: Increasing the temperature of a reaction typically increases the rate of reaction. This is because an increase in temperature increases the kinetic energy of particles, causing them to move faster and collide more frequently. The higher collision frequency leads to more successful collisions and an overall faster reaction rate.
Catalysts: Catalysts are substances that increase the rate of a reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy. This allows particles to overcome the energy barrier more easily, leading to a faster rate of reaction. Catalysts can also increase the number of successful collisions by bringing reactant particles close together.
Surface area: The rate of a reaction is also influenced by the surface area of the reactants. When reactants are in the form of solids, increasing the surface area by grinding or cutting them into smaller pieces exposes more particles to the surrounding medium. This increases the frequency of collisions and leads to a faster rate of reaction.
In conclusion, the rate of a chemical reaction can be affected by the concentration of reactants, temperature, catalysts, and surface area. Understanding these factors is important in controlling and optimizing reaction rates in various industrial and laboratory processes.
Concentration
Concentration is a key factor in determining the rate of a chemical reaction. It refers to the amount of a substance present in a given volume or space. In the context of rate of reaction, concentration can influence the number of collisions between reactant particles and therefore affect the reaction speed.
When the concentration of reactants is higher, there are more particles available for collisions. This increases the likelihood of successful collisions, leading to a faster reaction rate. On the other hand, when the concentration is lower, there are fewer particles available for collisions, resulting in a slower reaction rate.
Furthermore, concentration can also affect the reaction rate by altering the reaction equilibrium. In some reactions, an increase in reactant concentration can shift the equilibrium towards the products, leading to a higher reaction rate. Conversely, a decrease in concentration can shift the equilibrium towards the reactants, resulting in a lower reaction rate.
It is important to note that concentration is just one of the factors that can influence the rate of a chemical reaction. Other factors such as temperature, pressure, and the presence of catalysts also play a significant role. Understanding the relationship between concentration and reaction rate is crucial for scientists and engineers who need to control and optimize chemical processes in various industries, including pharmaceuticals, energy production, and materials manufacturing.
Temperature
The rate of reaction is affected by temperature. As the temperature increases, the rate of reaction generally increases. This is because an increase in temperature gives the particles more kinetic energy, causing them to move faster and collide with each other more frequently. This increases the chances of successful collisions and therefore increases the rate of reaction.
The effect of temperature on the rate of reaction can be explained by the collision theory. According to this theory, particles must collide with sufficient energy and in the correct orientation in order for a reaction to occur. When the temperature is increased, the particles gain more energy, which increases the likelihood of successful collisions. Additionally, an increase in temperature also leads to an increase in the average kinetic energy of the particles, which means that more particles will have enough energy to overcome the activation energy barrier and react.
Key terms:
- Rate of reaction: The speed at which reactants are converted into products in a chemical reaction.
- Temperature: A measure of the average kinetic energy of particles.
- Kinetic energy: The energy of an object due to its motion.
- Collision theory: The theory that states that particles must collide with sufficient energy and in the correct orientation in order for a reaction to occur.
- Activation energy: The minimum amount of energy required for a chemical reaction to occur.
In conclusion, temperature plays a crucial role in determining the rate of reaction. Increasing the temperature provides more kinetic energy to the particles, leading to an increase in the frequency and effectiveness of collisions. This results in a higher rate of reaction. Understanding the relationship between temperature and the rate of reaction is important in various fields, such as industrial processes, where controlling reaction rates is essential for optimizing productivity and efficiency.
Surface Area
The surface area of a substance plays a crucial role in determining the rate of reaction. In simple terms, surface area refers to the area of the solid that is in contact with the reactants or other substances. It is an essential factor in many chemical reactions as it affects the rate at which reactants can come into contact with each other.
When the surface area of a substance is increased, more particles of the substance are exposed to the reactants, leading to an increase in the rate of reaction. This is because a larger surface area provides a greater number of sites for reactant particles to collide and interact. As a result, the chances of successful collisions and the formation of products are enhanced.
For example, consider a block of solid substance and a solution that needs to react with the surface of that substance. If the block is in the form of a large cube, only the external surface of the cube will be in contact with the solution. However, if the cube is broken down into smaller pieces or powdered, the surface area exposed to the solution will be significantly increased. This will lead to a faster reaction as the solution can penetrate and react with a larger portion of the substance.
Additionally, surface area is also important in heterogeneous catalysis, where the catalyst is in a different phase from the reactants. The catalyst’s surface provides a platform for reactant molecules to adsorb and react, and a larger surface area allows for more potential reaction sites, increasing the rate of the catalytic reaction.
In conclusion, the surface area of a substance directly influences the rate of reaction. Increasing the surface area exposes more particles to reactants, leading to a higher rate of successful collisions and faster reaction. Understanding and controlling the surface area is essential for optimizing reaction conditions and improving reaction efficiency.
Catalysts
A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway. It does this by lowering the activation energy required for the reaction to occur. Catalysts are not consumed in the reaction and can be used repeatedly.
Catalysts work by interacting with the reactants to form an intermediate species, which then reacts to form the desired product. This intermediate species allows the reaction to occur at a lower energy level, making it easier for the reactants to overcome the energy barrier and react. The catalyst helps to stabilize the transition state of the reaction, facilitating the formation of the product.
Catalysts can be classified into two types: homogeneous catalysts and heterogeneous catalysts. Homogeneous catalysts exist in the same phase as the reactants, whereas heterogeneous catalysts are in a different phase. Homogeneous catalysts often involve the formation of complexes with the reactants, while heterogeneous catalysts typically function by adsorbing the reactants onto their surface.
Catalysts play a crucial role in many industrial processes, such as the production of fertilizers, polymers, and pharmaceuticals. They enable these reactions to occur at a faster rate, reducing the energy requirements and increasing the efficiency of the process. Additionally, catalysts can also help reduce the environmental impact of chemical reactions by allowing them to occur under milder conditions.
In conclusion, catalysts are substances that facilitate chemical reactions by lowering the activation energy. They provide an alternative reaction pathway, increasing the rate of the reaction without being consumed in the process. Catalysts are used in various industries to improve the efficiency of processes and reduce the energy requirements. Their ability to accelerate reactions and operate under milder conditions makes them valuable tools in the field of chemistry.
How to measure the rate of reaction
Measuring the rate of reaction is crucial in understanding the kinetics of chemical reactions. It allows scientists to determine how fast reactants are consumed or products are formed, providing valuable information about the reaction’s speed and efficiency. There are several methods used to measure the rate of reaction, each with its own advantages and limitations.
One common method is the initial rate method, which involves monitoring the reactants’ concentration at the beginning of the reaction. By measuring the change in concentration over a short period of time, scientists can calculate the initial rate of reaction. This method provides a snapshot of the reaction’s speed at the very start and can be useful when the reaction rate changes over time.
Another approach is the continuous monitoring method, where the change in concentration is measured continuously throughout the reaction. This method gives a more detailed picture of the reaction’s progress and allows scientists to observe any fluctuations in the rate. Spectroscopic techniques, such as UV-Vis spectroscopy, can be used to track the changes in absorbance or emission of the reactants or products, providing real-time data on the reaction rate.
In some cases, measuring the rate of reaction requires measuring other physical properties, such as pressure or volume. This is often the case in gas-phase reactions, where the ideal gas law can be used to relate the reaction rate to the change in pressure or volume. Gas chromatography and mass spectrometry are commonly employed techniques to measure the concentration of reactants and products in gas reactions.
Overall, measuring the rate of reaction is essential in understanding the dynamics of chemical reactions. By employing various methods, scientists can gather valuable data that can be used to optimize reaction conditions, design efficient catalytic systems, or investigate reaction mechanisms.
The Importance of Understanding Rate of Reaction
Understanding the rate of reaction is crucial in many areas of science and technology. Whether it’s studying the kinetics of chemical reactions, designing efficient industrial processes, or developing new drugs, a thorough understanding of how fast reactions occur and the factors that influence their rates is essential.
One of the main reasons why understanding rate of reaction is important is because it allows scientists to predict and control the outcomes of chemical reactions. By knowing the rate at which reactants are converted into products, researchers can optimize reaction conditions, such as temperature, pressure, and catalysts, to maximize the yield of desired products. This is especially critical in industrial processes, where efficiency and productivity are key factors for economic success.
The rate of reaction also provides important insights into the underlying mechanisms of chemical reactions. By studying how different factors, such as concentration, surface area, and temperature, affect the rate, scientists can gain a deeper understanding of the molecular events that occur during a reaction. This knowledge can then be used to develop new reaction pathways, improve reaction selectivity, and even discover new reaction mechanisms.
Furthermore, understanding rate of reaction is crucial in the field of pharmacology. The rate at which drugs are metabolized in the body determines their efficacy and duration of action. By studying the rate of drug metabolism and elimination, scientists can optimize drug dosages and administration schedules to ensure maximum therapeutic effect while minimizing side effects.
In conclusion, understanding the rate of reaction is of utmost importance in various scientific and technological endeavors. It enables scientists to predict and control reactions, gain insights into reaction mechanisms, and optimize processes in fields ranging from chemistry to pharmacology. Without a thorough understanding of rate of reaction, progress in these areas would be greatly hindered.