If you are studying chemistry and want to test your knowledge of redox reactions, a worksheet with answers is a useful tool. Redox reactions involve the transfer of electrons between reactants, and understanding them is essential in many areas of chemistry, including electrochemistry and organic chemistry.
A redox reaction worksheet with answers will typically include a series of questions that ask you to identify the oxidizing agent, the reducing agent, and the changes in oxidation numbers for each reactant. It may also ask you to balance the equation or determine the products of the reaction.
Working through a redox reaction worksheet can help you practice your skills in identifying redox reactions and understanding how electron transfer occurs. It can also help you improve your problem-solving skills and prepare for exams or quizzes. By providing answers to the questions, the worksheet allows you to check your work and understand where you may need additional practice.
In conclusion, a redox reaction worksheet with answers is a valuable resource for studying and practicing redox reactions. It allows you to test your knowledge, practice problem-solving, and gain a better understanding of how electrons are transferred in chemical reactions. Whether you are a student studying chemistry or a professional in the field, using a redox reaction worksheet can help you further your understanding and improve your skills.
Redox Reaction Worksheet with Answers PDF
Redox reactions, short for reduction-oxidation reactions, are a type of chemical reaction where electrons are transferred between species. These reactions play a crucial role in various processes, including metabolism, corrosion, and the production of energy. Understanding redox reactions is essential for students studying chemistry, as it helps them grasp the fundamental principles of chemical reactions.
One of the best ways to practice and reinforce the concepts of redox reactions is through worksheets. Redox reaction worksheets provide students with an opportunity to test their knowledge and skills in balancing equations and identifying oxidation numbers. These worksheets typically come with a set of questions and problems that require students to apply their understanding of redox reactions.
The Redox Reaction Worksheet with Answers PDF is a valuable resource for students and teachers alike. This PDF file contains a collection of redox reaction worksheets with detailed solutions, allowing students to check their answers and learn from their mistakes. The worksheets cover a range of topics, from basic redox reactions to more complex scenarios, such as balancing equations in acidic or basic solutions.
The Redox Reaction Worksheet with Answers PDF is structured in an easy-to-follow format. Each worksheet starts with a brief explanation of the topic, followed by a set of questions and problems. The solutions to these questions are provided at the end of the worksheet, allowing students to self-assess their understanding of redox reactions. This PDF file is an excellent supplementary resource for chemistry teachers, as it can be used for in-class activities, homework assignments, or exam preparation.
In conclusion, the Redox Reaction Worksheet with Answers PDF is a valuable tool for students studying redox reactions. It provides a comprehensive collection of worksheets that cover various aspects of redox reactions, allowing students to practice and reinforce their understanding of the topic. By using this resource, students can improve their problem-solving skills, enhance their knowledge of redox reactions, and ultimately excel in their chemistry studies.
The Basics of Redox Reactions
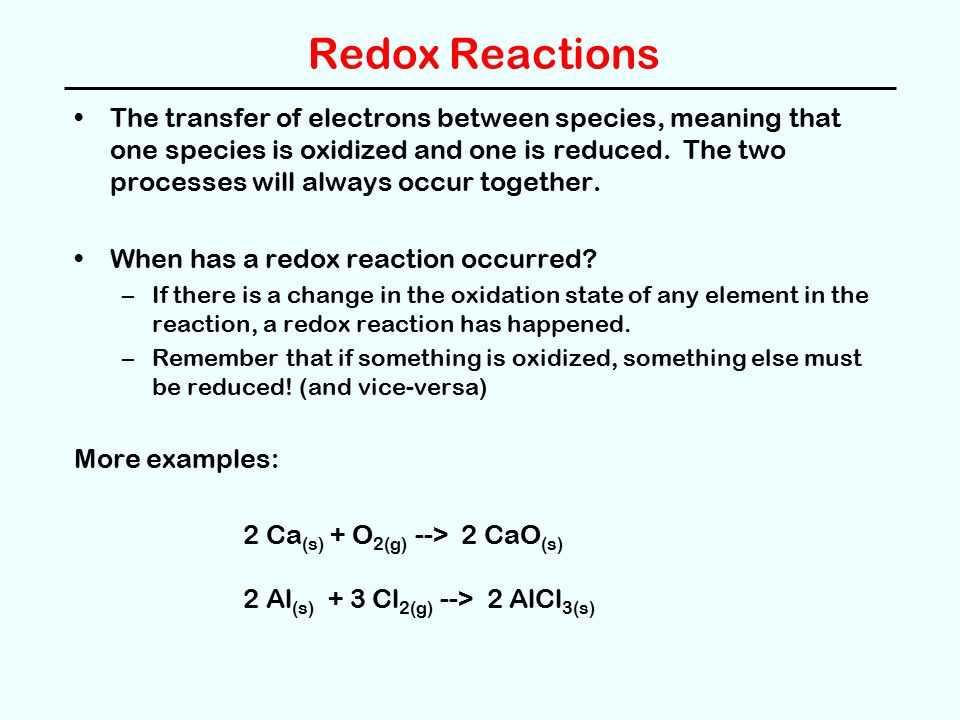
Redox (reduction-oxidation) reactions are fundamental chemical reactions that involve the transfer of electrons between atoms or molecules. These reactions play a crucial role in various biological, industrial, and environmental processes. Understanding the basics of redox reactions is essential for students and professionals in chemistry and related fields.
In a redox reaction, there is always an electron donor and an electron acceptor. The electron donor is oxidized (loses electrons), while the electron acceptor is reduced (gains electrons). This transfer of electrons allows for the conversion of chemical species from one oxidation state to another.
Redox reactions can be identified by changes in the oxidation numbers of atoms or molecules involved. Oxidation numbers are a way to track the transfer of electrons. An increase in oxidation number indicates oxidation, while a decrease indicates reduction. For example, in the reaction:
2Ag+ + Cu → 2Ag + Cu2+
Silver ions (Ag+) are being reduced to elemental silver (Ag), while copper is being oxidized from its elemental form to Cu2+ ions.
It is important to note that redox reactions always occur simultaneously – a substance cannot be oxidized without another being reduced. The total number of electrons lost by the oxidized species must equal the total number of electrons gained by the reduced species.
Furthermore, redox reactions can be classified into different types, such as combination, decomposition, displacement, and combustion reactions, based on the specific changes occurring.
In conclusion, redox reactions are essential for understanding the transfer of electrons in chemical processes. By identifying changes in oxidation numbers, one can determine the oxidized and reduced species in a reaction. These reactions have widespread applications and are crucial for various fields of study.
Identifying Redox Reactions

Redox reactions, also known as oxidation-reduction reactions, are chemical reactions that involve the transfer of electrons between reactant species. These reactions can be identified by observing changes in the oxidation states of atoms or ions in the reactants and products. In a redox reaction, one species undergoes oxidation, or an increase in oxidation state, while another species undergoes reduction, or a decrease in oxidation state.
To identify a redox reaction, it is important to first determine the oxidation states of all atoms or ions in the reactants and products. Oxidation states are assigned based on certain rules and can be represented by a positive or negative number. When the oxidation state of an atom or ion increases, it has undergone oxidation, while a decrease in oxidation state indicates reduction. By comparing the oxidation states of atoms or ions in the reactants and products, any changes can be identified.
In addition to changes in oxidation states, redox reactions also involve the transfer of electrons. The species that loses electrons is the reducing agent, while the species that gains electrons is the oxidizing agent. The reducing agent is oxidized and causes another species to be reduced, while the oxidizing agent is reduced and causes another species to be oxidized. By identifying the reducing and oxidizing agents, the redox nature of the reaction can be confirmed.
In summary, identifying redox reactions involves analyzing changes in the oxidation states of atoms or ions and determining the transfer of electrons between reactant species. By understanding these concepts and applying the rules for assigning oxidation states, it is possible to identify and classify chemical reactions as redox reactions.
Balancing Redox Reactions
Redox reactions involve the transfer of electrons between different chemical species. Balancing these reactions is important to ensure that the number of atoms and charge is conserved. There are several methods that can be used to balance redox reactions, including the half-reaction method and the oxidation number method.
The half-reaction method involves separating the reaction into two half-reactions: the oxidation half-reaction, where electrons are lost, and the reduction half-reaction, where electrons are gained. By balancing the number of atoms and charge in each half-reaction, the overall redox reaction can be balanced. This method is often used for reactions that occur in acidic or basic solutions.
The oxidation number method involves assigning oxidation numbers to each element in the reaction and then balancing the changes in oxidation numbers. The element that undergoes oxidation will have an increase in its oxidation number, while the element that undergoes reduction will have a decrease in its oxidation number. By adjusting the coefficients of the reactants and products, the overall redox reaction can be balanced. This method is particularly useful for reactions that occur in neutral or basic solutions.
Example:
Let’s take the reaction between iron (Fe) and chlorine (Cl2) as an example:
Fe + Cl2 → FeCl3
In this reaction, iron is oxidized from an oxidation state of 0 to +3, while chlorine is reduced from an oxidation state of 0 to -1. To balance this reaction using the half-reaction method, we can first separate it into two half-reactions:
- Oxidation half-reaction: Fe → Fe^3+ + 3e^-
- Reduction half-reaction: Cl2 + 2e^- → 2Cl^-
By balancing the number of atoms and charge in each half-reaction, we get:
- Oxidation half-reaction: 2Fe → 2Fe^3+ + 6e^-
- Reduction half-reaction: 3Cl2 + 6e^- → 6Cl^-
Multiplying the oxidation half-reaction by 3 and the reduction half-reaction by 2, we can combine them to get the balanced overall redox reaction:
6Fe + 3Cl2 → 6FeCl3
By following these balancing methods, redox reactions can be accurately balanced to ensure the conservation of atoms and charge. This is crucial for understanding chemical reactions and their implications in various scientific disciplines, such as chemistry and biochemistry.
Half-Reactions and Oxidation States

In redox reactions, the transfer of electrons between species is facilitated through the use of half-reactions. A half-reaction is a representation of the oxidation or reduction process of a specific species. Each half-reaction is balanced separately and then combined to give the overall balanced redox equation.
To determine the half-reactions and oxidation states, it is important to first identify the species being oxidized and reduced in the reaction. The species being oxidized will typically have an increase in oxidation state, while the species being reduced will have a decrease in oxidation state.
The oxidation state of an element in a compound can be determined using a set of guidelines. For example, in a neutral compound, the sum of the oxidation states of all the elements is equal to zero. In an ion, the sum of the oxidation states equals the charge of the ion. Elements in their elemental form have an oxidation state of zero, and the oxidation state of hydrogen is typically +1 while oxygen is typically -2.
Once the oxidation states of the species in the reaction are determined, the half-reactions can be written. The half-reaction for oxidation involves the species losing electrons, while the half-reaction for reduction involves the species gaining electrons. These half-reactions are balanced by adding appropriate coefficients to ensure that the number of atoms and charges are the same on both sides of the equation.
In summary, half-reactions and oxidation states are important tools for understanding and balancing redox reactions. By identifying the species being oxidized and reduced and determining their oxidation states, we can write balanced half-reactions that can be combined to give the overall balanced equation.
Redox Reactions in Everyday Life
Redox reactions, also known as oxidation-reduction reactions, play a crucial role in our daily lives. These reactions involve the transfer of electrons between different substances, resulting in the oxidation of one substance and the reduction of another. Redox reactions are essential for many processes, from the metabolism of food in our bodies to the generation of electricity in batteries.
One common example of a redox reaction in everyday life is the process of corrosion. When iron is exposed to oxygen and moisture, it undergoes oxidation, forming iron oxide (rust). This reaction is a classic example of a redox reaction, as the iron is oxidized, losing electrons, while the oxygen is reduced, gaining electrons. Corrosion can be seen on metal objects, such as cars or bicycles, over time.
Another example of a redox reaction is the use of bleach as a disinfectant. Bleach contains sodium hypochlorite, which acts as an oxidizing agent. When bleach comes into contact with bacteria or other organic matter, it oxidizes the substances, effectively killing the microorganisms. This oxidation-reduction reaction is responsible for the disinfecting properties of bleach.
Redox reactions are also involved in the process of respiration in living organisms. During cellular respiration, glucose is oxidized to produce energy, while oxygen is reduced to form water. This energy is then used by cells for various metabolic processes. Without redox reactions, the process of respiration and energy production would not be possible.
In conclusion, redox reactions are ubiquitous in our everyday lives. Whether it’s the rusting of metal, the disinfection of surfaces, or the production of energy in our bodies, redox reactions are happening all around us. Understanding these reactions can help us comprehend the world around us and find practical applications in various fields, from medicine to engineering.
Redox Reaction Worksheet: Practice Questions
Are you looking to practice your knowledge of redox reactions? This worksheet is designed to help you sharpen your skills by providing a series of practice questions. By working through these questions, you can solidify your understanding of redox reactions and improve your problem-solving abilities in this area.
Question 1:
Balance the following redox equation:
Fe2+(aq) + MnO4-(aq) → Fe3+(aq) + Mn2+(aq)
Question 2:
Identify the species that is being reduced and the species that is being oxidized in the following reaction:
2Na(s) + Cl2(g) → 2NaCl(s)
Question 3:
In the reaction below, determine the change in oxidation number for each element:
2KClO3(s) → 2KCl(s) + 3O2(g)
Question 4:
Calculate the oxidation number of sulfur in the compound H2SO4.
Question 5:
Balance the following redox equation:
KMnO4(aq) + HCl(aq) → MnCl2(aq) + KCl(aq) + Cl2(g) + H2O(l)
Use these practice questions to test your understanding of redox reactions and improve your problem-solving skills in this area. Refer to the answer key to check your answers and identify any areas that may require further study.