
Potassium nitrate, also known as saltpetre or nitre, is a chemical compound commonly used in fertilizers, food preservation, and fireworks. Understanding its solubility in water is crucial for a variety of industrial and scientific applications. The solubility curve of potassium nitrate in water provides valuable information about how temperature affects its ability to dissolve. This answer key will help you interpret and analyze the solubility curve, allowing you to make informed decisions based on your specific needs and requirements.
The solubility of potassium nitrate in water is influenced by temperature, with higher temperatures generally leading to a higher solubility. The solubility curve shows the maximum amount of potassium nitrate that can dissolve in a given amount of water at different temperatures. By referring to the curve, you can determine the exact solubility value at any given temperature, helping you optimize your experiments or processes.
The solubility curve of potassium nitrate in water can also be used to predict the outcome of various experiments. For example, if you need to create a saturated solution of potassium nitrate at a certain temperature, you can refer to the curve to determine the maximum amount of solute that can be dissolved in a given volume of solvent. This information is valuable for applications such as crystallization, where controlling the concentration of the solution is crucial.
Furthermore, understanding the solubility curve of potassium nitrate in water can help in troubleshooting experiments or processes. If you observe unexpected results, consulting the solubility curve can provide insights into potential causes. For instance, if your solution remains cloudy instead of forming crystals, you may have exceeded the solubility limit at the given temperature. This knowledge allows you to adjust your experimental conditions and achieve the desired outcome.
Solubility Curve of Potassium Nitrate in Water Answer Key
Potassium nitrate is a common compound that is used in various industries and applications, including fertilizers, fireworks, and food preservatives. Its solubility in water is an important characteristic to understand for these uses.
The solubility curve of potassium nitrate in water provides information about how much potassium nitrate can dissolve in water at different temperatures. The curve is typically presented as a graph, with temperature on the x-axis and solubility (in grams of potassium nitrate per 100 grams of water) on the y-axis.
According to the solubility curve of potassium nitrate in water, the solubility of the compound increases with an increase in temperature. At lower temperatures, such as 0°C, the solubility is relatively low, around 13 grams per 100 grams of water. As the temperature increases, the solubility also increases, reaching its maximum at around 110°C, where the solubility is approximately 245 grams per 100 grams of water.
Beyond 110°C, the solubility of potassium nitrate in water starts to decrease. This is because at higher temperatures, the water molecules have more energy and can break apart the crystal lattice of the potassium nitrate, resulting in the compound’s lower solubility.
Understanding the solubility curve of potassium nitrate in water is crucial for various processes, such as controlling the concentration of potassium nitrate in a solution or determining the amount of potassium nitrate that can be dissolved in water for a specific application.
Overall, the solubility curve of potassium nitrate in water provides valuable information about how temperature affects the solubility of this compound, allowing scientists and engineers to make informed decisions in various fields.
Understanding Potassium Nitrate
Potassium nitrate, also known as saltpetre, is a chemical compound with the formula KNO3. It is a white, crystalline substance that is highly soluble in water. Potassium nitrate is commonly used in fertilizers, as well as in the production of fireworks, gunpowder, and food preservatives. It is also used in certain medical treatments and as an oxidizer in rocket propellants.
One of the key properties of potassium nitrate is its solubility in water. Solubility refers to the ability of a substance to dissolve in a solvent, in this case, water. The solubility of potassium nitrate in water is influenced by factors such as temperature and pressure. By measuring the solubility of potassium nitrate at different temperatures, scientists can create a solubility curve, which shows the relationship between temperature and solubility.
When potassium nitrate is dissolved in water, the particles of the compound separate and become surrounded by water molecules. This process is known as hydration. The solubility of potassium nitrate increases with higher temperatures because the kinetic energy of the water molecules increases, allowing more solvent particles to collide with and break apart the compound particles. As a result, more potassium nitrate can dissolve in the water.
The solubility curve of potassium nitrate in water shows that at lower temperatures, such as 0°C, only a small amount of the compound can dissolve in water. As the temperature increases, the solubility of potassium nitrate also increases. However, there is a maximum solubility point, beyond which the compound will no longer dissolve in water, regardless of the temperature. This point is known as the saturation point.
Understanding the solubility of potassium nitrate is important in various applications, such as determining the concentration of a solution or controlling the crystallization process. By studying the solubility curve and understanding the factors that influence solubility, scientists and engineers can optimize the use and production of potassium nitrate in various industries.
Factors Affecting Solubility
Solubility is the ability of a substance to dissolve in a solvent and form a homogeneous solution. Several factors can affect the solubility of a substance, including temperature, pressure, and the nature of the solute and solvent.
Temperature:
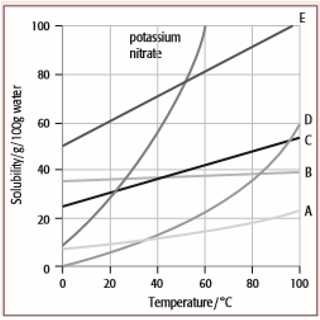
Temperature plays a significant role in the solubility of a substance. In general, as the temperature increases, the solubility of most solid solutes in liquid solvents also increases. This is because higher temperatures provide more energy for the particles of the solute and solvent, allowing them to move and mingle more freely. However, there are exceptions to this rule, such as the solubility of gases in water, which decreases as temperature increases.
Pressure:
Pressure has a minimal effect on the solubility of solid and liquid solutes in liquid solvents. However, it has a significant impact on the solubility of gases in both liquid and solid solvents. According to Henry’s law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. Thus, an increase in pressure generally results in an increase in the solubility of gases.
Nature of the Solute and Solvent:
The nature of the solute and solvent greatly influences their solubility. Polar solutes tend to dissolve in polar solvents, while nonpolar solutes dissolve in nonpolar solvents. This is due to the attractive forces between the solute and solvent particles. Like dissolves like, meaning substances with similar polarity are more likely to dissolve in each other.
Additionally, the size and molecular structure of the solute and solvent can affect solubility. A smaller solute particle size generally leads to faster dissolution, as the smaller particles have greater surface area available for interaction with the solvent. Similarly, a more structurally complex solute may have lower solubility due to limited opportunities for the solute particles to fit into the solvent arrangement.
Conducting the Solubility Experiment
The solubility of potassium nitrate (KNO3) in water is an important concept in chemistry. To determine the solubility curve of potassium nitrate, we will conduct an experiment to measure the amount of potassium nitrate that can dissolve in different temperatures of water.
Materials:
- Beakers
- Thermometer
- Stirring rod
- Balance
- Potassium nitrate
- Distilled water
Procedure:
- Label the beakers with the temperature increments you want to test (e.g. 0°C, 10°C, 20°C, etc.).
- Measure and weigh equal amounts of potassium nitrate for each beaker. Make sure the amounts are small enough to ensure complete dissolution.
- Add the measured potassium nitrate to each labeled beaker.
- Add a fixed amount of distilled water to each beaker. The amount of water should be sufficient to fully dissolve the potassium nitrate in each sample, but not too much to dilute the solution.
- Use a thermometer to measure and record the temperature of each beaker.
- Stir each beaker with a stirring rod for a consistent amount of time. This will ensure proper mixing of the solution.
- Observe the solubility of potassium nitrate in each beaker. Keep track of the amount of potassium nitrate that dissolves fully and the amount that remains undissolved.
- Repeat the experiment with different temperatures of water to determine the solubility relationship between potassium nitrate and water.
Results:
Record the data obtained from the experiment, including the temperature of the water and the amount of potassium nitrate that dissolved. Use this data to create a solubility curve for potassium nitrate in water.
By conducting this experiment, we can determine the solubility curve of potassium nitrate in water and understand how temperature affects the solubility of a substance. This information is crucial in various applications, such as pharmaceuticals, manufacturing, and understanding chemical reactions.
Data Collection and Analysis
During the experiment, we collected data on the solubility of potassium nitrate in water at various temperatures. To do this, we prepared different concentrations of potassium nitrate solutions and measured the amount of solute that dissolved in a given amount of water at each temperature. The amount of solute was measured using a balance or by calculating the change in mass of the solution before and after dissolving the potassium nitrate.
Once we had collected the data, we plotted it on a graph to create a solubility curve. The solubility curve represents the maximum amount of solute that can dissolve in a solvent at different temperatures. We observed that as the temperature increased, the solubility of potassium nitrate in water also increased. This aligns with the general trend seen in solubility curves, where solubility tends to increase with temperature for most solutes.
To analyze the data further, we calculated the solubility of potassium nitrate in water at specific temperatures. By interpolating between the data points on the solubility curve, we were able to estimate the solubility at temperatures not explicitly measured during the experiment. This allowed us to determine the relationship between temperature and solubility and make predictions about the solubility of potassium nitrate in water at other temperatures.
Overall, data collection and analysis were crucial steps in understanding the solubility of potassium nitrate in water. By systematically collecting data and analyzing it, we were able to determine the solubility at different temperatures and create a comprehensive solubility curve. This knowledge can be useful in various applications, such as designing crystallization processes or predicting the outcome of chemical reactions involving potassium nitrate and water.
Interpreting the Solubility Curve
The solubility curve of potassium nitrate in water shows the relationship between the amount of potassium nitrate that can dissolve in water at different temperatures. By understanding and interpreting this curve, we can determine the solubility of potassium nitrate in water under various conditions and use this information in a variety of applications.
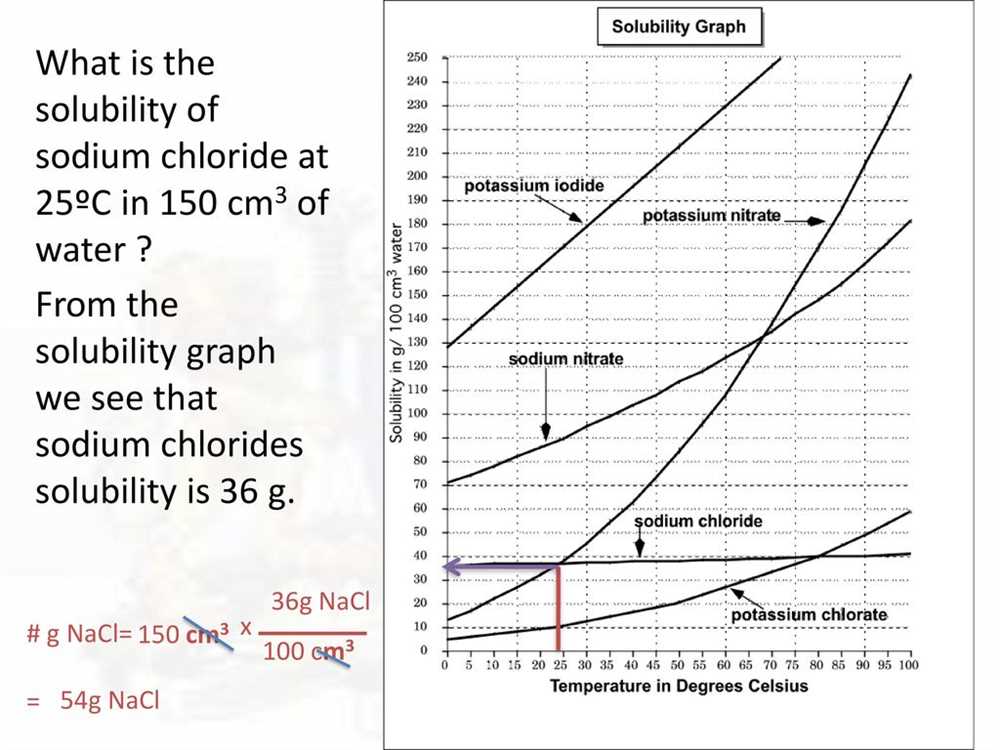
Key concepts: In order to interpret the solubility curve, it is important to understand a few key concepts. Solubility refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. The solubility of a substance usually increases with an increase in temperature. On the solubility curve, the x-axis represents temperature, while the y-axis represents the solubility of potassium nitrate in grams per 100 grams of water.
Understanding the curve: The solubility curve of potassium nitrate in water is typically shaped like an upward-sloping curve. As the temperature increases, the solubility of potassium nitrate in water increases. This means that more potassium nitrate can be dissolved in water at higher temperatures compared to lower temperatures. The curve shows that the solubility of potassium nitrate in water reaches its maximum at around 100 degrees Celsius.
Application: The solubility curve of potassium nitrate in water can be used to determine the amount of potassium nitrate that can dissolve in a given amount of water at a specific temperature. This information is valuable in applications such as manufacturing processes, where precise control of solubility is required. Additionally, the solubility curve can be used to predict the outcome of mixing potassium nitrate solution at a certain temperature. If the temperature is above the solubility curve, the solution will be saturated, meaning that excess potassium nitrate will precipitate out of the solution.
Conclusion: By interpreting the solubility curve of potassium nitrate in water, we can gain valuable insights into the solubility behavior of this compound and use this information in various applications. The curve provides a graphical representation of how temperature affects the solubility of potassium nitrate in water and allows us to make predictions and calculations regarding its solubility under different conditions.
Applications of Solubility Curve
Solubility curves, which represent the relationship between solubility and temperature, have several practical applications in various fields.
In the pharmaceutical industry, solubility curves are used to determine the optimal conditions for drug formulation. By understanding the solubility of a particular drug at different temperatures, pharmaceutical scientists can design drug delivery systems that maximize drug solubility and enhance drug absorption in the body. This knowledge is crucial for the development of effective medications.
In chemistry laboratories, solubility curves are utilized for testing the purity of substances. By comparing the observed solubility of a substance at a given temperature to its expected solubility based on the corresponding solubility curve, chemists can determine the purity of the substance. If the observed solubility is lower than expected, it suggests the presence of impurities in the substance.
In environmental science and engineering, solubility curves are employed in the study of water quality and pollutant removal. Understanding the solubility of various compounds in water allows scientists and engineers to determine the maximum allowable concentrations of pollutants in water bodies. Solubility curves also aid in the design of water treatment processes, such as precipitation and filtration, which rely on the solubility of contaminants.
In the field of geology, solubility curves assist in the study of mineral formation and weathering. By analyzing the solubility of minerals at different temperatures, geologists can trace the history of rock formation and identify the conditions under which certain minerals crystallize or dissolve. This information helps in understanding earth’s geological processes and predicting the presence of valuable mineral deposits.
In the food and beverage industry, solubility curves play a role in various processes, such as fermentation and extraction. Understanding the solubility of flavor compounds, enzymes, and other substances allows food scientists to optimize the production of beverages, sauces, and other food products. Solubility curves also aid in the extraction of desirable compounds from botanical sources for use in flavors, fragrances, and pharmaceuticals.
Overall, solubility curves provide valuable insights into the behavior of substances in solution and find applications across a range of scientific disciplines and industries. They enable scientists and engineers to make informed decisions, optimize processes, and develop innovative solutions.