Stoichiometry is a fundamental concept in chemistry that revolves around the quantitative relationships between reactants and products in a chemical reaction. Chapter 9 of any stoichiometry course delves deeper into this topic, requiring students to not only understand the basic principles but also apply them to various scenarios.
In the quest to master stoichiometry, students often come across Chapter 9 tests that serve as checkpoints of their knowledge and understanding. These tests include a range of questions that assess their ability to balance chemical equations, calculate the quantities of reactants and products, and utilize conversion factors efficiently.
When it comes to finding answers to these Chapter 9 test questions, students are often on the lookout for reliable sources that can provide them with step-by-step solutions. This article aims to be that source, offering detailed answers to a selection of Chapter 9 test questions in stoichiometry.
By going through these answers, students can not only check the accuracy of their own solutions but also gain a better understanding of the reasoning and calculations required to solve such problems. With these insights, students can build a solid foundation in stoichiometry, paving the way for success in future chemistry endeavors.
Chapter 9 Test Answers for Stoichiometry
In chapter 9 of the stoichiometry course, students were tested on their understanding of various concepts related to chemical reactions and the calculations involved. The test included questions on balancing chemical equations, determining the limiting reactant and percent yield, as well as calculating the molar mass and stoichiometric ratios.
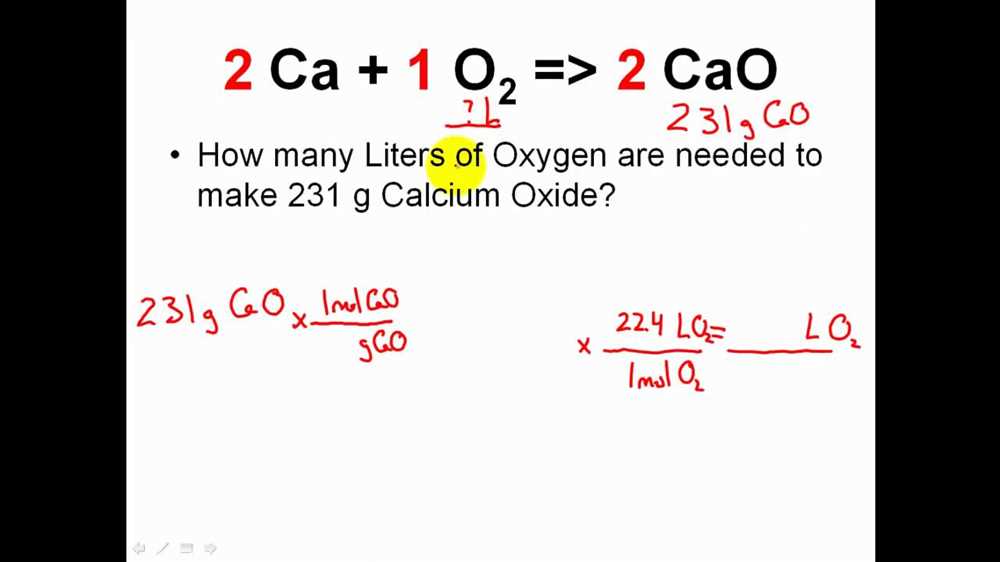
Question 1: Balance the following chemical equation: 2H2 + O2 → 2H2O
The correct answer is:
- 2H2 + O2 → 2H2O
Question 2: A reaction produces 25.0 grams of CaCO3. Calculate the molar mass of CaCO3.
The molar mass of CaCO3 is calculated as follows:
- Ca: 1 atom x 40.08 g/mol = 40.08 g/mol
- C: 1 atom x 12.01 g/mol = 12.01 g/mol
- O: 3 atoms x 16.00 g/mol = 48.00 g/mol
Adding up the individual masses gives:
- 40.08 g/mol + 12.01 g/mol + 48.00 g/mol = 100.09 g/mol
Question 3: Determine the percent yield if 25.0 grams of CO2 is produced from the reaction and the theoretical yield is calculated as 30.0 grams.
The percent yield is calculated as follows:
- Percent yield = (Actual yield / Theoretical yield) x 100%
- Percent yield = (25.0 g / 30.0 g) x 100%
Performing the calculation gives:
- (25.0 g / 30.0 g) x 100% = 83.33%
Overall, the chapter 9 test in stoichiometry focused on assessing students’ understanding of balancing equations, calculating molar masses, and determining percent yields. These concepts are fundamental in understanding and applying stoichiometric calculations to real-world chemical reactions.
Overview of Stoichiometry
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It is based on the principle of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Stoichiometry allows chemists to determine the amount of reactants needed to produce a certain amount of product, or the amount of product that can be obtained from a given amount of reactants. It involves the use of balanced chemical equations, which show the mole ratio between reactants and products.
In stoichiometry, the mole ratio can be used to convert between different units, such as moles, grams, and liters. This allows chemists to perform calculations that help them understand and predict the outcome of chemical reactions.
Stoichiometry calculations often involve the use of stoichiometric coefficients, which represent the number of moles of each substance involved in a balanced chemical equation. These coefficients can be used to determine the limiting reactant, which is the reactant that is completely consumed in a reaction and determines the amount of product that can be formed.
Overall, stoichiometry is a fundamental concept in chemistry that allows scientists to understand and quantify the relationships between reactants and products in chemical reactions. It plays a crucial role in a wide range of applications, from determining the efficiency of industrial chemical processes to understanding biological reactions in living organisms.
Basics of Chemical Equations
Chemical equations are an essential part of understanding and predicting chemical reactions. They represent the starting materials, known as reactants, on the left side of the equation and the resulting products on the right side. Each element and compound is represented by its chemical formula consisting of symbols and subscripts.
Reactants are the substances that combine together to undergo a chemical reaction. They are written on the left side of the equation. Reactants can be elements, compounds, or ions, and their chemical formulas are used to represent them. For example, in the equation H2 + O2 → H2O, H2 and O2 are the reactants.
Products are the substances that are formed as a result of the chemical reaction. They are written on the right side of the equation. Like reactants, products can be elements, compounds, or ions, and their chemical formulas are used to represent them. In the above equation, H2O is the product.
Chemical equations must be balanced, meaning that the number of atoms of each element on both sides of the equation must be equal. This is achieved by adjusting the coefficients, which represent the number of molecules or formula units of each substance. For example, in the equation 2H2 + O2 → 2H2O, the coefficient of H2 is 2, indicating that two molecules of H2 are required to react with one molecule of O2 to produce two molecules of H2O.
Understanding the basics of chemical equations is crucial for stoichiometry, as it allows us to calculate the quantities of reactants and products involved in a chemical reaction. By balancing the equation and using stoichiometric coefficients, we can determine the precise ratios and amounts of substances participating in the reaction.
Balancing Chemical Equations

Balancing chemical equations is a fundamental concept in chemistry that involves ensuring that the number of atoms of each element on the reactant side is equal to the number of atoms on the product side. This process is essential to accurately represent chemical reactions and the conservation of mass.
When balancing a chemical equation, it is important to start by identifying the reactants and products involved in the reaction. Each element in the equation needs to have the same number of atoms on both sides. To achieve this balance, coefficients can be added in front of the reactants or products to adjust the number of atoms of each element.
For example, let’s consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). The equation initially written as H2 + O2 → H2O is not balanced since there are two hydrogen atoms on the reactant side but only one hydrogen atom on the product side. To balance the equation, the coefficient 2 can be added in front of the water molecule, resulting in the balanced equation: 2H2 + O2 → 2H2O.
In more complex equations, it may be necessary to balance multiple elements simultaneously. This can be done by focusing on one element at a time and adjusting the coefficients accordingly. Additionally, it is important to check that the final balanced equation satisfies the law of conservation of mass, which states that the total mass of the reactants must be equal to the total mass of the products.
Steps to balance chemical equations:
- Identify the reactants and products in the equation.
- Count the number of atoms of each element on both sides of the equation.
- Start by balancing elements that appear in only one molecule on each side.
- Balance elements that appear in multiple molecules by adjusting coefficients.
- Continue balancing until the number of atoms of each element is equal on both sides.
- Check that the final balanced equation satisfies the law of conservation of mass.
Balancing chemical equations is a skill that requires practice and understanding of chemical formulas and the behavior of different elements. It is an essential aspect of chemistry and allows scientists to accurately represent and predict the outcomes of chemical reactions.
Stoichiometric Calculations
Stoichiometry is a branch of chemistry that deals with calculating the quantitative relationships between reactants and products in chemical reactions. It is a fundamental concept in chemistry as it allows scientists to predict the amounts of substances that are involved in a chemical reaction.
Stoichiometric calculations involve using balanced chemical equations to determine the molar ratios between reactants and products. These calculations can be used to determine the amount of reactant needed to produce a certain amount of product, or vice versa.
To perform stoichiometric calculations, it is necessary to know the balanced chemical equation for the reaction, as well as the molar masses of the substances involved. The balanced equation provides the molar ratios between reactants and products, while the molar masses allow for the conversion between mass and moles.
Stoichiometric calculations often involve converting between grams, moles, and liters. This requires the use of conversion factors, which are derived from the molar ratios in the balanced chemical equation. These conversion factors allow for the cancellation of units and ensure that the calculation is performed correctly.
In addition to determining the amount of reactant or product in a chemical reaction, stoichiometric calculations can also be used to calculate percent yield, which is a measure of the efficiency of a reaction. Percent yield is calculated by comparing the actual yield of a reaction to the theoretical yield, which is the amount predicted by stoichiometry.
Overall, stoichiometric calculations are an essential tool in chemistry that allow scientists to quantitatively analyze and predict the outcomes of chemical reactions. They provide valuable information about the amounts of substances involved in a reaction and can be used to optimize reaction conditions and maximize yield.
Limiting Reactants
In stoichiometry, the concept of limiting reactants plays a crucial role in determining the amount of product that can be formed in a chemical reaction. A limiting reactant is the reactant that is completely consumed in the reaction, thereby limiting the amount of product that can be formed.
When two or more reactants are involved in a reaction, it is important to determine which reactant is the limiting reactant in order to accurately calculate the amount of product. This can be done by comparing the stoichiometric ratio between the reactants and the balanced chemical equation.
To determine the limiting reactant, one can begin by calculating the number of moles of each reactant. This can be done by dividing the given mass of each reactant by its molar mass. Once the number of moles is determined, the stoichiometric ratio can be used to determine the number of moles of the other reactant that would be required to completely react with the limiting reactant.
The reactant that requires a greater amount of the other reactant is the limiting reactant, as it will be completely consumed in the reaction. The amount of product that can be formed is then determined by the moles of the limiting reactant and the stoichiometric ratio between the limiting reactant and the product.
In summary, the concept of limiting reactants allows us to determine the maximum amount of product that can be formed in a chemical reaction. By comparing the stoichiometric ratio and the number of moles of each reactant, we can identify the limiting reactant and accurately calculate the amount of product.
Percent Yield

In chemistry, percent yield is used to determine the efficiency of a chemical reaction. It is a measurement of how much product is obtained compared to the maximum possible amount that could be obtained based on the reactants used. Percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100%. The theoretical yield is the amount of product that would be obtained if the reaction proceeded perfectly, without any losses or side reactions.
To calculate percent yield, you first need to determine the actual yield of the reaction. This is the amount of product that is actually obtained in the laboratory. It can be measured using various techniques such as weighing the product or analyzing it using spectroscopy. The actual yield is then divided by the theoretical yield, which is calculated based on the stoichiometry of the reaction and the amounts of reactants used. The resulting value is multiplied by 100% to get the percent yield.
Percent yield is an important concept in chemistry because it allows chemists to assess the efficiency of a reaction and identify any potential sources of error or loss. A high percent yield indicates that the reaction is efficient and produces a high amount of product, while a low percent yield suggests that there may be limitations or inefficiencies in the reaction. It is often used to compare different reaction conditions or to optimize reaction parameters in order to achieve the highest possible yield.
Factors that can affect percent yield include impurities in the reactants, side reactions, incomplete conversion of reactants, losses during product isolation or purification, and experimental errors. By understanding and controlling these factors, chemists can strive to improve the percent yield of a reaction and maximize the production of desired products.
Example:
Suppose a chemical reaction is expected to produce 50 grams of product based on the stoichiometry of the reaction. However, when the reaction is carried out in the laboratory, only 40 grams of product are obtained. To calculate the percent yield, you would divide the actual yield (40 grams) by the theoretical yield (50 grams) and multiply by 100%. So the percent yield would be 80%. This means that the reaction is 80% efficient in terms of producing the desired product.
Note:
- The percent yield can never be greater than 100%. If the actual yield is greater than the theoretical yield, it means there is an error in the experimental procedure or measurement.
- Percent yield calculations are based on the assumption that the reaction is carried out under ideal conditions and that the stoichiometry of the reaction is known accurately.