
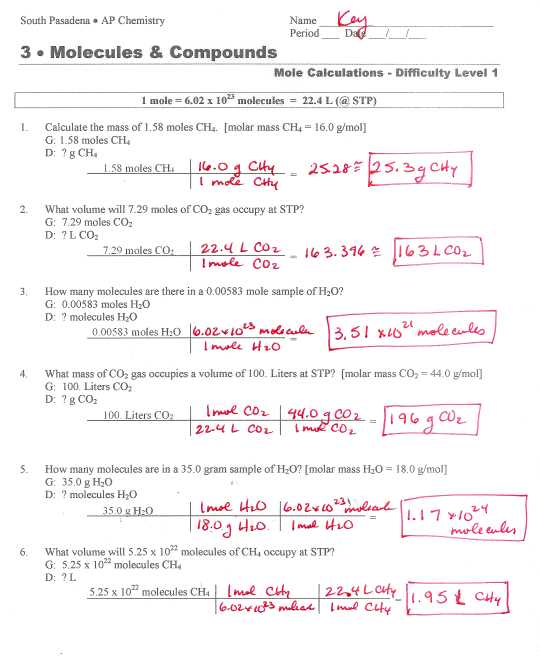
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between the reactants and products in chemical reactions. One important aspect of stoichiometry is the conversion of moles to mass, which is crucial for determining the amounts of substances involved in a reaction.
When solving stoichiometry problems, it is important to have a clear understanding of the balanced chemical equation and the molar ratios between the reactants and products. These ratios allow us to convert between the number of moles of a substance and its mass.
A stoichiometry mole to mass problems worksheet provides various scenarios where students are required to convert the given number of moles of a substance to its corresponding mass. These problems help students develop a solid understanding of the concept and reinforce their knowledge of stoichiometric calculations.
The answers to the stoichiometry mole to mass problems worksheet depend on the specific scenario and the given information. By using the molar mass of the substance, which is found by adding up the atomic masses of its constituent elements, students can calculate the mass corresponding to the given number of moles.
In conclusion, stoichiometry mole to mass problems worksheet answers involve converting the number of moles of a substance to its corresponding mass using the molar mass. These problems are an essential part of stoichiometry and help students develop their problem-solving skills and understanding of chemical reactions.
Section 1: What is stoichiometry?
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It is often described as the calculation of quantities in chemical reactions. It allows us to determine the amount of reactants needed or the amount of products produced in a chemical reaction.
In stoichiometry, we use balanced chemical equations to relate the number of moles of reactants and products. A balanced chemical equation provides the ratios of moles of reactants and products involved in the reaction. These ratios can be used to calculate the mass of reactants consumed or the mass of products formed.
Stoichiometry is based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants must be equal to the total mass of the products. Stoichiometry allows us to determine the precise amounts of reactants and products involved in a chemical reaction, which is essential for understanding and predicting chemical reactions.
By using stoichiometric calculations, chemists can determine the theoretical yield of a reaction, which is the maximum amount of product that can be obtained from a given amount of reactants. These calculations help to optimize chemical processes, determine the efficiency of a reaction, and ensure that reactions are carried out with the appropriate quantities of reactants.
Understanding the Concept

In stoichiometry, mole to mass problems are used to calculate the mass of a substance based on the number of moles present. This concept is essential in chemistry as it allows us to determine the quantity of reactants and products in a chemical reaction.
To solve mole to mass problems, we must first understand the relationship between moles and mass. The molar mass of a substance is the mass of one mole of that substance. It is calculated by summing the atomic masses of all the atoms in a molecule.
To convert moles to mass, we use the equation:
mass (g) = moles × molar mass (g/mol)
Let’s consider an example. If we have 2 moles of water (H2O), we can calculate the mass of water using the molar mass of water: 18.02 g/mol. Using the formula mentioned above:
mass (g) = 2 mol × 18.02 g/mol = 36.04 g
Therefore, 2 moles of water would have a mass of 36.04 grams.
It is important to note that stoichiometry calculations involve using balanced chemical equations to determine the mole ratios between different substances. These ratios allow us to convert between moles of different substances, which is crucial in solving mole to mass problems.
Section 2: Mole to Mass Conversion
In stoichiometry, mole to mass conversions are used to determine the mass of a substance based on the number of moles of that substance. This conversion is essential in chemical calculations, as it allows scientists to calculate the amount of a particular substance needed or produced in a chemical reaction.
To convert from moles to mass, you need to know the molar mass of the substance. The molar mass is the mass of one mole of that substance and is usually expressed in grams per mole (g/mol). It can be calculated by summing the atomic masses of all the atoms in the chemical formula of the substance.
To perform the mole to mass conversion, you can use the following steps:
- Determine the number of moles of the substance given in the problem.
- Identify the molar mass of the substance.
- Multiply the number of moles by the molar mass to calculate the mass of the substance.
For example, if you are given 2 moles of carbon dioxide (CO2), you can calculate its mass by multiplying the number of moles (2) by the molar mass of carbon dioxide (44.01 g/mol). The result would be 88.02 grams of carbon dioxide.
It is important to note that when performing mole to mass conversion, you should pay attention to significant figures. The number of significant figures in the given number of moles should be reflected in the calculated mass.
Explaining the Process
Molar mass is an important concept in stoichiometry, as it allows us to convert between the number of moles of a substance and its mass. To solve stoichiometry problems that involve converting from moles to mass, we follow a specific process.
First, we start with the given amount of the substance in moles. This can usually be found in the problem statement. Next, we use the molar mass of the substance to convert from moles to grams. The molar mass is the mass of one mole of the substance, which can be calculated by summing the atomic masses of all the atoms in the chemical formula.
Once we have the mass of the substance in grams, we can use this value to convert to any other desired unit, such as kilograms or milligrams, if needed. To do this, we simply use the appropriate conversion factor based on the desired units.
Let’s work through an example to illustrate the process. Suppose we have 2.5 moles of carbon dioxide (CO2). First, we find the molar mass of carbon dioxide: 12.01 g/mol (for carbon) + 2(16.00 g/mol) (for oxygen) = 44.01 g/mol. Now, we can use this molar mass to convert the moles of carbon dioxide to grams: 2.5 moles CO2 * 44.01 g/mol = 110.025 g CO2. Finally, if we wanted to convert this mass to kilograms, we would divide by 1000: 110.025 g CO2 / 1000 = 0.110025 kg CO2.
In summary, converting from moles to mass in stoichiometry problems involves finding the molar mass of the substance, converting moles to grams using the molar mass, and then possibly converting to other units if necessary. This process allows us to relate the amount of a substance in moles to its corresponding mass in grams or other units.
Section 3: Basic mole to mass problems
In this section, we will explore the concept of converting moles to mass in stoichiometry problems. This is an important calculation in chemistry as it allows us to determine the mass of a substance based on its mole quantity.
When solving mole to mass problems, it is essential to use the molar mass of the substance. The molar mass represents the mass of one mole of the substance and is expressed in grams per mole (g/mol).
To convert moles to mass, you need to follow these simple steps:
- Determine the number of moles given in the problem.
- Identify the molar mass of the substance.
- Multiply the number of moles by the molar mass to obtain the mass in grams.
Let’s consider an example: If you have 3 moles of carbon dioxide (CO2), you would multiply this by the molar mass of CO2 (44.01 g/mol) to obtain the mass. The calculation would be as follows: 3 moles x 44.01 g/mol = 132.03 grams. Therefore, 3 moles of carbon dioxide would have a mass of 132.03 grams.
It is important to note that when solving stoichiometry problems, you must always consider the balanced chemical equation to determine the appropriate molar mass to use. Additionally, be sure to round your final answer to the appropriate number of significant figures.
Step-by-step examples of Stoichiometry mole to mass problems
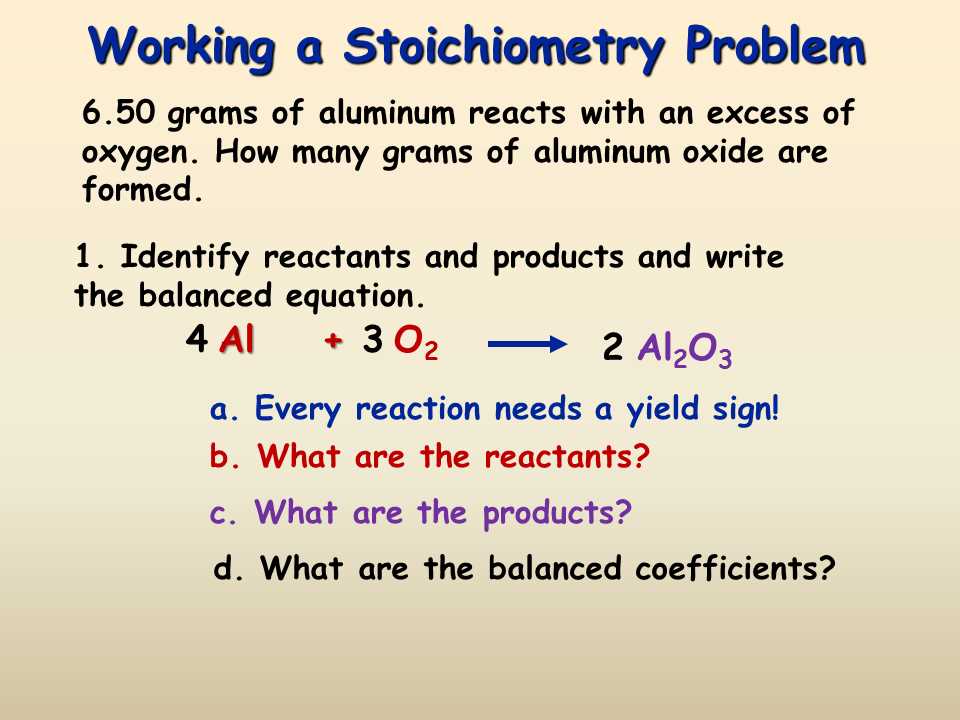
Stoichiometry involves using balanced chemical equations to determine the relationships between the amounts of reactants and products. In mole to mass problems, we are given the number of moles and asked to find the mass of a substance involved in a chemical reaction. Here are some step-by-step examples to help you understand how to solve these types of problems.
Example 1:
Calculate the mass of sodium chloride (NaCl) that can be produced from 2 moles of sodium (Na) reacting with excess chlorine (Cl2).
- Write the balanced chemical equation: 2Na + Cl2 -> 2NaCl
- Convert moles of Na to moles of NaCl using the stoichiometric ratio from the balanced equation. In this case, the ratio is 2 moles of NaCl : 2 moles of Na.
- Convert moles of NaCl to grams of NaCl using the molar mass of NaCl (22.99 g/mol for Na and 35.45 g/mol for Cl).
The mass of NaCl that can be produced from 2 moles of Na is 117.38 g.
Example 2:
Find the mass of water (H2O) produced from the reaction of 5 moles of hydrogen (H2) with excess oxygen (O2).
- Write the balanced chemical equation: 2H2 + O2 -> 2H2O
- Convert moles of H2 to moles of H2O using the stoichiometric ratio from the balanced equation. In this case, the ratio is 2 moles of H2O : 2 moles of H2.
- Convert moles of H2O to grams of H2O using the molar mass of H2O (2.02 g/mol for H and 16.00 g/mol for O).
The mass of H2O produced from 5 moles of H2 is 180.18 g.
These step-by-step examples demonstrate how to solve stoichiometry mole to mass problems by using balanced chemical equations, stoichiometric ratios, and molar masses. It’s important to remember to convert between moles and mass using the molar mass of each element or compound involved in the reaction. Practice these examples and apply the same approach to solve similar problems.
Section 4: Stoichiometry mole to mass problems worksheet
The stoichiometry mole to mass problems worksheet is a valuable tool for students to practice their understanding of converting between moles and masses in chemical reactions. This section of the worksheet focuses specifically on these types of problems and provides students with the opportunity to apply their knowledge in a variety of scenarios.
One example problem from the worksheet asks students to calculate the mass of potassium nitrate (KNO3) needed to produce a certain number of moles of nitrogen gas (N2) according to the balanced chemical equation. In order to solve this problem, students must first determine the molar ratio between potassium nitrate and nitrogen gas, and then use this ratio to convert the given number of moles of nitrogen gas into moles of potassium nitrate. Finally, they can convert the moles of potassium nitrate into grams by multiplying by the molar mass of potassium nitrate.
Another example problem involves determining the mass of oxygen gas (O2) produced from the decomposition of a certain mass of potassium chlorate (KClO3). Students must first calculate the number of moles of potassium chlorate using its molar mass, and then use the balanced chemical equation to determine the molar ratio between potassium chlorate and oxygen gas. Finally, they can convert the moles of oxygen gas into grams by multiplying by the molar mass of oxygen gas.
By completing these types of problems on the stoichiometry mole to mass problems worksheet, students will become more confident in their ability to convert between moles and masses in chemical reactions. This skill is crucial for understanding and predicting the outcomes of chemical reactions, and is essential for success in chemistry courses and further study in the field of science.
Providing a worksheet for practice
One effective way to reinforce the concepts of stoichiometry and mole to mass problems is by providing students with a worksheet for practice. This allows them to apply their understanding of the topic to a variety of problems and strengthen their skills.
The worksheet should be designed in a way that progressively increases in difficulty, starting with basic mole to mass conversions and gradually incorporating more complex scenarios. It should include a mix of multiple-choice, fill-in-the-blank, and calculation-based questions to cater to different learning styles.
Additionally, the worksheet should provide clear instructions and step-by-step explanations for each question to help students understand the reasoning behind the calculations. This will enhance their problem-solving skills and build confidence in their ability to solve stoichiometry problems.
It can also be beneficial to include sample problems with solutions at the end of the worksheet, allowing students to compare their own answers and identify any errors. This will facilitate self-assessment and highlight areas that may require further review or clarification.
By providing a well-structured and comprehensive worksheet for practice, educators can give students the opportunity to reinforce their understanding of stoichiometry mole to mass problems and develop confidence in their ability to tackle similar problems in the future.
Section 5: Answers to mole to mass problems worksheet
In this section, we will provide the answers to the mole to mass problems worksheet. These problems involve calculating the mass of a given substance based on its molar quantity.
1. How many grams are in 0.5 moles of carbon dioxide (CO2)?
To calculate the mass of carbon dioxide, we need to determine the molar mass of CO2. The molar mass of carbon dioxide is calculated by adding the atomic masses of carbon and oxygen:
Molar mass of carbon dioxide = (atomic mass of carbon) + 2 * (atomic mass of oxygen)
Using the atomic masses from the periodic table, we find:
Molar mass of carbon dioxide = 12.01 g/mol + 2 * 16.00 g/mol = 44.01 g/mol
Next, we can calculate the mass of 0.5 moles of carbon dioxide using the equation:
Mass = (number of moles) * (molar mass)
Mass = 0.5 moles * 44.01 g/mol = 22.01 grams
Therefore, there are 22.01 grams in 0.5 moles of carbon dioxide.
2. What is the mass of 2.5 moles of water (H2O)?
To calculate the mass of water, we need to determine the molar mass of H2O. The molar mass of water is calculated by adding the atomic masses of hydrogen and oxygen:
Molar mass of water = 2 * (atomic mass of hydrogen) + (atomic mass of oxygen)
Using the atomic masses from the periodic table, we find:
Molar mass of water = 2 * 1.01 g/mol + 16.00 g/mol = 18.02 g/mol
Next, we can calculate the mass of 2.5 moles of water using the equation:
Mass = (number of moles) * (molar mass)
Mass = 2.5 moles * 18.02 g/mol = 45.05 grams
Therefore, the mass of 2.5 moles of water is 45.05 grams.
3. How many grams are in 1 mole of potassium chloride (KCl)?
To calculate the mass of potassium chloride, we need to determine the molar mass of KCl. The molar mass of potassium chloride is calculated by adding the atomic masses of potassium and chlorine:
Molar mass of potassium chloride = (atomic mass of potassium) + (atomic mass of chlorine)
Using the atomic masses from the periodic table, we find:
Molar mass of potassium chloride = 39.10 g/mol + 35.45 g/mol = 74.55 g/mol
Therefore, there are 74.55 grams in 1 mole of potassium chloride.
These are the answers to the mole to mass problems worksheet. By using the molar masses of the substances, we can calculate the mass of a given quantity of the substance in moles.