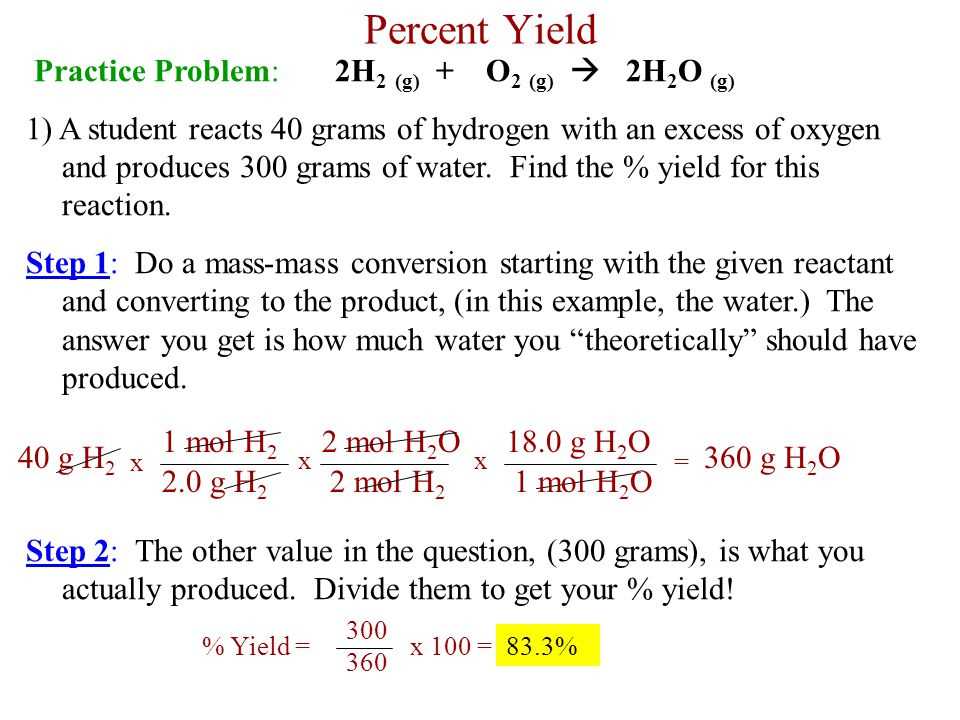
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows chemists to determine the ideal ratios between substances, as well as calculate the amount of product formed or reactant consumed.
If you’re studying stoichiometry and need some extra practice, you’ve come to the right place. In this article, we will provide you with the answers to the Stoichiometry Practice 1 Worksheet. This comprehensive guide will walk you through each question, explaining the steps and calculations involved in finding the correct answer.
Whether you’re a student preparing for a chemistry exam or a teacher looking for resources to help your students, this guide will be an invaluable tool. It will not only provide you with the answers to the worksheet but also offer a detailed breakdown of the solution process. By understanding the concepts and calculations involved in stoichiometry, you’ll be better equipped to tackle any stoichiometry problem you encounter.
So, let’s dive in and explore the answers to the Stoichiometry Practice 1 Worksheet. Get ready to enhance your understanding of stoichiometry and improve your problem-solving skills in chemistry!
Stoichiometry Practice 1 Worksheet Answers
In stoichiometry, we use balanced chemical equations to determine the relationships between the amounts of reactants and products in a chemical reaction. The stoichiometry practice 1 worksheet provides a set of questions and problems to test our understanding of stoichiometry. Here are the answers to some of the problems:
1. How many moles of oxygen gas (O2) are needed to completely react with 2 moles of methane (CH4)?
To determine the number of moles of oxygen gas needed, we use the balanced chemical equation for the combustion of methane:
CH4 + 2O2 → CO2 + 2H2O
Based on the equation, we can see that 1 mole of methane reacts with 2 moles of oxygen gas. Therefore, if we have 2 moles of methane, we would need 4 moles of oxygen gas.
2. What mass of sulfuric acid (H2SO4) is produced when 25 grams of hydrogen gas (H2) reacts according to the following equation:
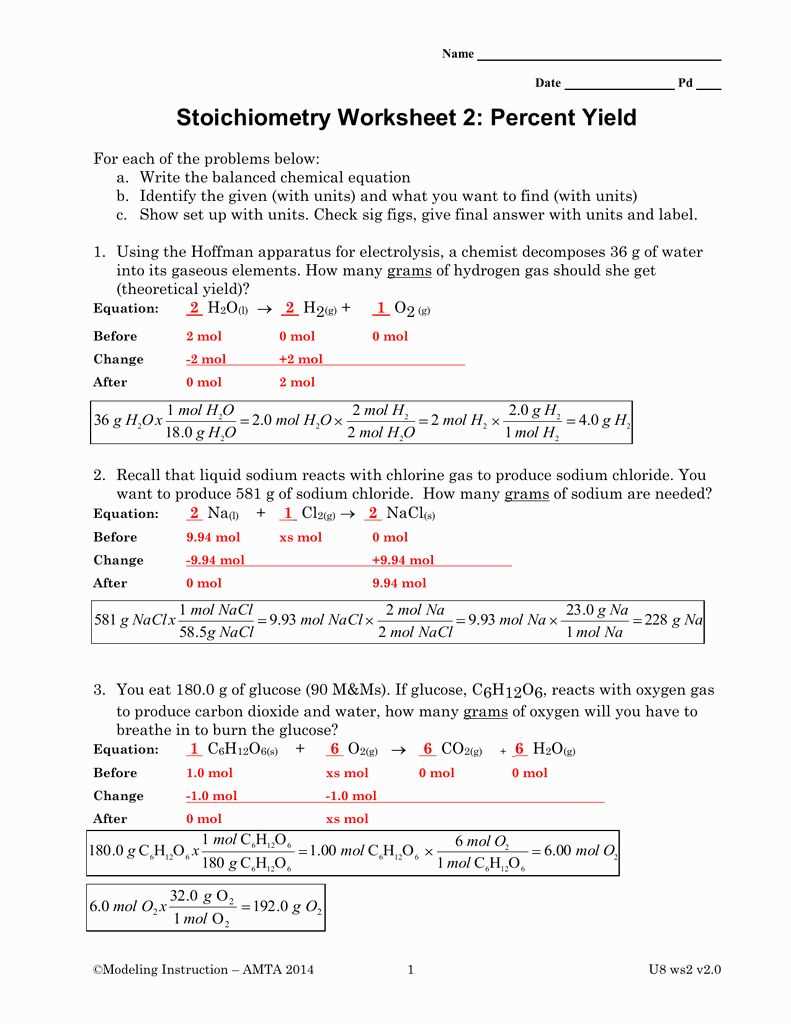
2H2 + O2 → 2H2O
First, we need to determine the number of moles of hydrogen gas present. Since the molar mass of hydrogen gas is 2 g/mol, we can calculate the number of moles using the formula: moles = mass/molar mass.
moles of hydrogen gas = 25 g / 2 g/mol = 12.5 mol
By looking at the balanced equation, we can see that 2 moles of hydrogen gas reacts to form 2 moles of water. Therefore, the moles of water produced would also be 12.5 mol.
Next, we need to convert the moles of water to the mass of sulfuric acid using its molar mass. The molar mass of sulfuric acid (H2SO4) is 98 g/mol.
mass of sulfuric acid = moles of water x molar mass of sulfuric acid
mass of sulfuric acid = 12.5 mol x 98 g/mol = 1225 g
Therefore, 1225 grams of sulfuric acid would be produced when 25 grams of hydrogen gas reacts.
These are just two examples of the types of questions that may be found in the stoichiometry practice 1 worksheet. By using the balanced chemical equations and the molar masses of the substances involved, we can calculate the quantities of reactants and products in a chemical reaction. This allows us to understand the stoichiometry and the relationship between the amounts of substances in a reaction.
What is Stoichiometry?

Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows us to calculate the amounts of substances involved in a reaction, predict the yield of products, and determine the limiting reactant.
In a chemical equation, the coefficients represent the ratio of molecules or moles of reactants and products. By using stoichiometric calculations, we can convert between grams, moles, and particles of a substance, and determine the amounts of substances needed or produced in a reaction. This is crucial for understanding and optimizing chemical reactions in industries such as pharmaceuticals, materials, and energy production.
Stoichiometry involves several key concepts and calculations, including balancing chemical equations, calculating molar ratios, determining the limiting reactant, and solving stoichiometric problems. It requires a strong understanding of the periodic table, atomic mass, molar mass, and chemical formulas.
To solve stoichiometric problems, we often use a step-by-step approach called dimensional analysis or the factor-label method. This involves converting between units using conversion factors derived from the coefficients in the balanced equation and the molar masses of substances.
- Balancing chemical equations: This step ensures that the number of atoms on both sides of the equation is equal.
- Calculating molar ratios: Molar ratios are used to convert between moles of different substances in the reaction.
- Determining the limiting reactant: The limiting reactant is the one that runs out first and determines the maximum amount of product that can be formed.
- Solving stoichiometric problems: These problems involve using the information from a balanced equation and the given quantities to calculate the desired quantities.
Overall, stoichiometry is a fundamental concept in chemistry that allows us to understand and manipulate chemical reactions on a quantitative level. It plays a critical role in various fields and industries and helps scientists and engineers design and optimize chemical processes.
Key Concepts and Formulas in Stoichiometry
In stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions, several key concepts and formulas are essential to solve problems and determine the amounts of substances involved. By understanding these concepts and formulas, chemists can accurately predict the yield of a reaction, determine the limiting reactant, and calculate the stoichiometric ratio.
Molar Mass: One of the fundamental concepts in stoichiometry is the molar mass of a substance, which is the mass of one mole of that substance. It is expressed in units of grams per mole (g/mol).
Avogadro’s Number: This key concept in chemistry represents the number of particles (atoms, molecules, ions, etc.) present in one mole of a substance. Avogadro’s number is approximately 6.022 × 10^23 particles/mol.
- Stoichiometric Coefficients: These coefficients, represented by the numbers in front of the chemical formulas in a balanced equation, indicate the molar ratios between reactants and products. They allow chemists to determine the stoichiometric ratio between different substances in a reaction.
- Mole-Mole Ratios: These ratios are derived from the stoichiometric coefficients and provide the relationship between moles of one substance and moles of another substance in a chemical reaction. By applying mole-mole ratios, chemists can convert between the amounts of reactants and products.
- Limiting Reactant: The limiting reactant is the substance that is completely consumed in a chemical reaction, limiting the amount of product that can be formed. To determine the limiting reactant, chemists compare the stoichiometric ratios and the available amounts of reactants.
- Theoretical and Actual Yield: The theoretical yield is the maximum amount of product that can be obtained based on the stoichiometry of the reaction. The actual yield is the amount of product obtained in a laboratory experiment. The percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100%, expressing the efficiency of the reaction.
By applying these key concepts and formulas in stoichiometry, chemists can accurately predict and calculate the quantitative aspects of chemical reactions, enabling them to optimize reaction conditions, understand reaction mechanisms, and design effective synthesis routes.
Step-by-Step Approach to Stoichiometry Problems
Stoichiometry is a branch of chemistry that deals with the calculation of the quantities of reactants and products involved in a chemical reaction. It is essential in understanding and predicting the outcome of chemical reactions. When solving stoichiometry problems, it is important to follow a step-by-step approach to ensure accuracy and clarity.
Step 1: Balanced Chemical Equation
The first step in solving a stoichiometry problem is to write and balance the chemical equation for the reaction. This equation represents the reactants and products involved in the reaction, and their respective molar ratios. Balancing the equation ensures that the law of conservation of mass is obeyed.
Step 2: Converting Units
Next, it is necessary to convert the given units to moles. This step requires using the molar mass of the given substance. The molar mass is the mass of one mole of a substance and can be calculated by summing the atomic masses of all the elements in the formula. Once the given quantity is converted to moles, it can be used in the calculation of the desired quantity.
Step 3: Mole Ratio
After converting the given quantity to moles, it is necessary to determine the mole ratio between the given substance and the desired substance in the equation. The mole ratio can be obtained from the coefficients in the balanced chemical equation. This ratio allows for the conversion between different substances in the reaction.
Step 4: Calculating Desired Quantity
The final step is to calculate the desired quantity of the substance. This can be done by using the mole ratio and the given quantity of the substance. The mole ratio allows for the conversion between moles of different substances, and the given quantity provides the starting point for the calculation. By multiplying the given quantity by the appropriate mole ratio, the desired quantity can be determined.
By following this step-by-step approach, stoichiometry problems can be solved accurately and efficiently. It is important to carefully consider each step and double-check calculations to ensure correct results. Stoichiometry is an essential skill for any chemistry student and is used in various applications, such as determining the amount of reactants needed for a specific reaction or predicting the yield of a reaction.
Practice Problems for Stoichiometry 1 Worksheet
In stoichiometry, we use balanced chemical equations to determine the relationship between the amounts of reactants and products involved in a chemical reaction. This helps us calculate the quantities of substances involved in the reaction, such as the mass, moles, or volume. To master stoichiometry, it is essential to practice solving various problems using the stoichiometric ratios.
Problem 1:
Given the balanced equation: 2H₂ + O₂ → 2H₂O
- If we have 4 moles of H₂, how many moles of O₂ are needed to completely react?
- What is the mass of H₂O produced when 1.5 moles of O₂ reacts?
Problem 2:
Consider the reaction: 3Fe + 4H₂O → Fe₃O₄ + 4H₂
- What mass of Fe is required to produce 180 g of Fe₃O₄?
- If we have 6 moles of H₂O, how many moles of Fe₃O₄ can be produced?
These practice problems aim to reinforce the understanding of stoichiometry calculations. By solving such problems, students can improve their ability to apply stoichiometric ratios in various scenarios and gain confidence in performing stoichiometry calculations. Practice is key to mastering stoichiometry and being able to solve complex problems in chemistry.
Detailed Solutions and Explanations for Practice Problems
In this section, we will provide detailed solutions and explanations for the practice problems on stoichiometry.
Problem 1:
We are given the following balanced chemical equation:
H2 + Cl2 → 2HCl
We are asked to determine the amount of HCl that can be formed from 5 moles of H2.
Solution:
According to the balanced chemical equation, 1 mole of H2 reacts with 1 mole of Cl2 to produce 2 moles of HCl.
Therefore, if we have 5 moles of H2, we can form 10 moles of HCl.
Problem 2:
We are given the following balanced chemical equation:
CaCO3 → CaO + CO2
We are asked to determine the mass of CaO produced from 25 grams of CaCO3.
Solution:
According to the balanced chemical equation, 1 mole of CaCO3 produces 1 mole of CaO.
The molar mass of CaCO3 is 100.09 g/mol.
Using the molar mass, we can determine the number of moles of CaCO3 in 25 grams:
Number of moles = mass / molar mass = 25 / 100.09 = 0.249 mol
Since 1 mole of CaCO3 produces 1 mole of CaO, the mass of CaO produced will also be 0.249 grams.
These were just a few examples to illustrate the process of solving stoichiometry problems. Remember to always balance the chemical equations, convert given quantities to moles, and use the coefficients in the balanced equation to determine the ratios of substances involved in the reaction.
Common Mistakes to Avoid in Stoichiometry Problems

In stoichiometry problems, it is common for students to make certain mistakes that can lead to incorrect answers. By being aware of these common mistakes, you can improve your accuracy and understanding of stoichiometry concepts. Here are some common mistakes to avoid:
1. Mixing up the coefficients in the balanced equation:
One of the most crucial steps in stoichiometry problems is writing a balanced equation. Make sure you accurately copy the coefficients from the balanced equation, as each coefficient represents the number of moles of a specific substance. Mixing up the coefficients can lead to incorrect calculations.
2. Not converting units properly:
Stoichiometry involves converting between different units, such as moles, grams, and liters. It is essential to properly convert units to ensure accurate calculations. Pay attention to the given units and use appropriate conversion factors to convert to the desired units.
3. Forgetting to account for stoichiometric ratios:
The stoichiometric ratios in a balanced equation determine the mole-to-mole relationships between substances. Forgetting to account for these ratios can result in incorrect calculations. Always check that you are using the correct stoichiometric ratios when performing stoichiometry calculations.
4. Ignoring significant figures:
Significant figures are important in stoichiometry problems as they indicate the precision of your answer. Ignoring significant figures can lead to inaccurate results. Always pay attention to significant figures and round your final answer to the appropriate number of significant figures.
5. Failing to double-check calculations:

Mistakes can easily occur in stoichiometry calculations, so it is important to double-check your work. Make sure you have accurately performed all the necessary calculations and have used the correct values and conversion factors. Taking the time to review your work can help you catch any mistakes before submitting your answer.
By avoiding these common mistakes, you can improve your accuracy in stoichiometry problems and develop a better understanding of the concepts involved. Practice and thoroughness are key in mastering stoichiometry calculations.