
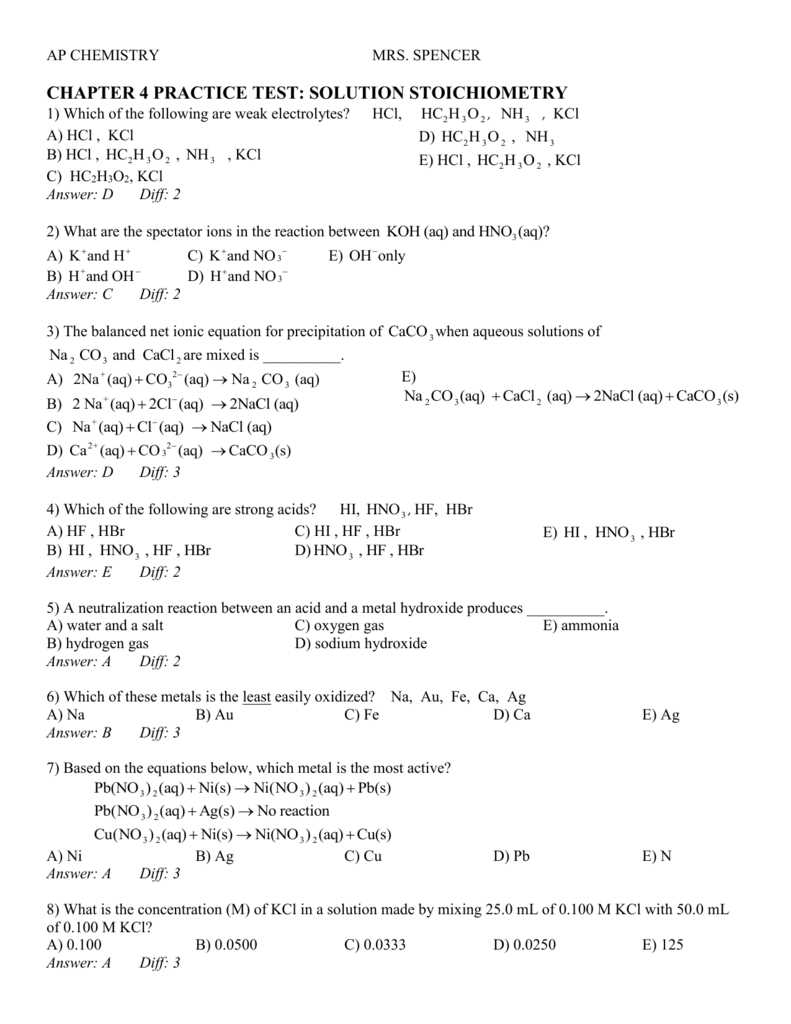
Stoichiometry is a crucial skill in chemistry that allows us to understand and predict the outcome of chemical reactions. It involves using balanced chemical equations to determine the quantities of substances involved, as well as the ratios in which they react. To truly master stoichiometry, it is essential to practice solving various problems and applying the principles to real-life scenarios.
A stoichiometry practice test is an excellent way to test your understanding of the subject and identify areas for improvement. It consists of a series of questions that require you to apply stoichiometric concepts, such as mole-to-mole ratios, mass-to-mass conversions, and reacting species. By practicing these problems, you can enhance your problem-solving skills and gain confidence in your ability to tackle more complex stoichiometry problems.
This practice test is designed to cover a wide range of stoichiometry problems, including single and double replacement reactions, limiting reactant calculations, percent yield, and empirical formulas. Each question is crafted to challenge your knowledge and critical thinking skills, helping you develop a deep understanding of stoichiometry and its practical applications. Whether you are a student preparing for an exam or a chemistry enthusiast looking to sharpen your skills, this practice test will provide you with ample opportunities to practice and refine your stoichiometric abilities.
What is stoichiometry?
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between the reactants and products in chemical reactions. It allows chemists to determine how much of each reactant is needed and how much of each product is produced in a chemical reaction. By understanding stoichiometry, chemists can predict and calculate the amounts of substances involved in a reaction, as well as the outcomes of various chemical reactions.
In stoichiometry, the key concept is the mole ratio, which is the ratio of the number of moles of each substance in a balanced chemical equation. This ratio allows chemists to convert between the mass of a substance and the number of moles, and vice versa. By using stoichiometric calculations, chemists can determine the limiting reactant, the theoretical yield, and the percent yield of a reaction.
Stoichiometry is important in many practical applications, such as in the field of medicine and pharmaceuticals, where it is used to determine the correct quantities of reactants needed to synthesize a desired compound. It is also used in environmental chemistry to study pollutant reactions and in the production of chemicals and materials in the industrial sector. Overall, stoichiometry is a fundamental concept in chemistry that plays a crucial role in understanding and predicting chemical reactions.
The Importance of Stoichiometry in Chemistry
Stoichiometry is a fundamental concept in chemistry that plays a crucial role in understanding chemical reactions and predicting the amounts of products and reactants involved. It allows chemists to determine the relationships between the quantities of substances involved in a chemical reaction, based on the balanced equation.
Stoichiometry is important in chemistry because it provides a quantitative understanding of chemical reactions. It helps chemists calculate the exact amounts of reactants needed to obtain a desired amount of product and vice versa. This knowledge is essential in various fields of chemistry, including pharmaceuticals, materials science, and environmental studies.
In pharmaceuticals, stoichiometry is vital for drug synthesis and analysis. Chemists need to accurately determine the stoichiometry of reactants and products to ensure the efficacy and safety of the drugs being developed. Understanding the stoichiometry of reactions also helps in optimizing reaction conditions to maximize yield and minimize waste.
Stoichiometry is also important in materials science, where it is used to determine the composition and properties of different substances. By understanding the stoichiometry of a compound, scientists can determine its exact chemical formula and predict its behavior under different conditions. This knowledge is crucial in designing new materials with specific properties, such as strength, conductivity, or reactivity.
In environmental studies, stoichiometry is used to understand and predict the impact of chemical reactions on ecosystems. By studying the stoichiometry of nutrient cycles, scientists can determine the availability of essential elements in an ecosystem and assess the potential for nutrient pollution or imbalance. This information is vital for managing and protecting natural resources and preventing environmental degradation.
In conclusion, stoichiometry is a fundamental concept in chemistry that is essential for understanding chemical reactions and predicting the quantities of substances involved. Its importance extends to various fields, including pharmaceuticals, materials science, and environmental studies, where knowledge of stoichiometry is crucial for synthesis, analysis, and understanding the behavior of substances.
Understanding the Concept of Stoichiometry

Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationship between reactants and products in a chemical reaction. It allows chemists to predict the amount of products formed or reactants consumed based on the balanced chemical equation. By understanding stoichiometry, scientists can determine the most efficient way to carry out a reaction and optimize the yield of desired products.
One important aspect of stoichiometry is the concept of the mole. A mole is a unit of measurement in chemistry that represents a specific number of particles, which is approximately 6.02 x 10^23. It is often used to convert between mass and the number of particles in a substance. The mole is used to determine the stoichiometric ratio between different compounds in a chemical reaction, allowing chemists to calculate the exact amounts of reactants needed or products produced.
In stoichiometry, reactions follow the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction, only rearranged. This means that the total mass of the reactants must be equal to the total mass of the products. By using the stoichiometric coefficients in a balanced chemical equation, chemists can calculate the mass of reactants needed or products produced.
Stoichiometry can also be used to determine limiting and excess reactants in a chemical reaction. The limiting reactant is the substance that is completely consumed in the reaction, while the excess reactant is the substance that is left over after the reaction is complete. By calculating the stoichiometric ratios and comparing the amounts of reactants, chemists can identify the limiting reactant and predict the maximum amount of product that can be obtained.
In conclusion, stoichiometry is a crucial concept in chemistry that allows scientists to understand and predict the quantitative relationships in chemical reactions. It provides a framework for calculating the amounts of reactants needed and products produced, optimizing reaction conditions, and determining the limiting reactants. By mastering the concept of stoichiometry, chemists can effectively design and control chemical reactions in various fields of research and industries.
Stoichiometry calculations using balanced chemical equations
Stoichiometry is an essential concept in chemistry that allows us to determine the quantities of reactants and products in a chemical reaction. It is based on the principle of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
To perform stoichiometry calculations, we first need a balanced chemical equation, which represents the reactants and products in the reaction. The coefficients in the balanced equation indicate the relative amounts of each substance involved in the reaction.
Using stoichiometry, we can determine various quantities such as the amount of reactant needed to produce a certain amount of product, or the amount of product that can be obtained from a given amount of reactant. This is done by using mole-to-mole ratios derived from the coefficients in the balanced equation.
For example, consider the reaction: 2H2 + O2 -> 2H2O. This balanced equation tells us that 2 moles of hydrogen gas react with 1 mole of oxygen gas to produce 2 moles of water. If we have 4 moles of hydrogen gas and want to know how many moles of water can be produced, we can use the mole-to-mole ratio to calculate it:
- 4 moles H2 * (2 moles H2O / 2 moles H2) = 4 moles H2O
Stoichiometry calculations are not limited to moles. We can also use stoichiometry to determine mass, volume, or any other unit as long as we have the appropriate conversion factors. These conversion factors are derived from the molar mass of each substance involved in the reaction.
In conclusion, stoichiometry calculations using balanced chemical equations allow us to determine the quantities of reactants and products in a chemical reaction. By using mole-to-mole ratios and conversion factors, we can easily convert between different units and obtain valuable information about the reaction.
Step-by-step guide to solving stoichiometry problems
Stoichiometry problems involve calculations that determine the relationships between reactants and products in a chemical reaction. By following a step-by-step approach, you can successfully solve these problems and better understand the underlying concepts.
Step 1: Identify the balanced chemical equation
The first step in solving a stoichiometry problem is to identify the balanced chemical equation for the reaction. Make sure to double-check that the equation is balanced with equal numbers of atoms on both sides.
Step 2: Determine the given and the desired
After identifying the balanced equation, determine what is given and what is desired in the problem. The given can be the mass, volume, or number of moles of a substance, while the desired is what you are trying to find.
Step 3: Convert the given to moles
Convert the given quantity (mass, volume, or number of particles) to moles using the appropriate conversion factor. This involves using the molar mass of the substance if dealing with mass, or the molar volume if dealing with volume.
Step 4: Use the stoichiometry ratios
Use the stoichiometry ratios from the balanced equation to establish a relationship between the given and the desired quantity. This involves comparing the coefficients of the substances in the equation to determine the mole ratio.
Step 5: Convert back to the desired quantity
Convert the moles obtained in the previous step to the desired quantity using the appropriate conversion factor. This may involve using the molar mass or molar volume, depending on the desired quantity.
Step 6: Check the answer and units
Finally, check your answer and ensure that it makes sense. Make sure to include the correct units in your final answer to ensure clarity and accuracy.
By following these steps and practicing regularly, solving stoichiometry problems can become easier and more intuitive. Keep in mind that practice is key to mastering this skill, so don’t hesitate to challenge yourself with a variety of problems.
Common types of stoichiometry problems
In chemistry, stoichiometry is a branch that deals with the quantitative relationships between the reactants and products in chemical reactions. It involves calculations and conversions based on balanced chemical equations. Stoichiometry problems can vary in complexity, but there are several common types that students often encounter.
1. Mole-to-Mole Calculations: One common type of stoichiometry problem involves determining the number of moles of one substance needed to react with a given number of moles of another substance, based on the balanced chemical equation.
2. Mass-to-Mass Calculations: This type of stoichiometry problem involves converting the mass of one substance to the mass of another substance in a chemical reaction. It requires using the molar mass of the substances and the balanced chemical equation.
3. Limiting Reactant Problems: In stoichiometry, limiting reactant problems involve determining which reactant will be completely consumed in a reaction and which reactant will be left over. This calculation is important because it determines the maximum amount of product that can be formed.
4. Percent Yield Calculations: Percent yield is a measure of the efficiency of a chemical reaction, calculated by comparing the actual yield (the amount of product obtained experimentally) to the theoretical yield (the amount of product calculated using stoichiometry). Percent yield problems involve calculating the percentage of the theoretical yield obtained.
- 5. Gas Stoichiometry Problems: Gas stoichiometry involves calculating the volumes or pressures of reactant or product gases using stoichiometric ratios and the ideal gas law.
These are just a few examples of the common types of stoichiometry problems encountered in chemistry. It is important for students to understand the principles behind stoichiometry and practice solving different problem types to develop proficiency in this area.
Tips and tricks for mastering stoichiometry
Stoichiometry, the calculation of the quantities of reactants and products in chemical reactions, can be a challenging concept to master. However, with the right approach and some helpful tips and tricks, you can improve your understanding and performance in stoichiometry problems. Here are some strategies to help you excel in this area of chemistry.
1. Understand the concept of moles
To solve stoichiometry problems, it is crucial to have a firm grasp of the concept of moles. Mole is a unit of measurement used in chemistry to express the amount of a substance. Knowing the relationship between moles and mass, as well as moles and volume, will greatly assist you in stoichiometric calculations.
2. Practice balancing chemical equations
One of the key steps in stoichiometry is balancing chemical equations. Being able to balance equations quickly and accurately is essential for determining the ratios of reactants and products. Dedicate time to practicing balancing chemical equations to develop this skill.
3. Utilize dimensional analysis
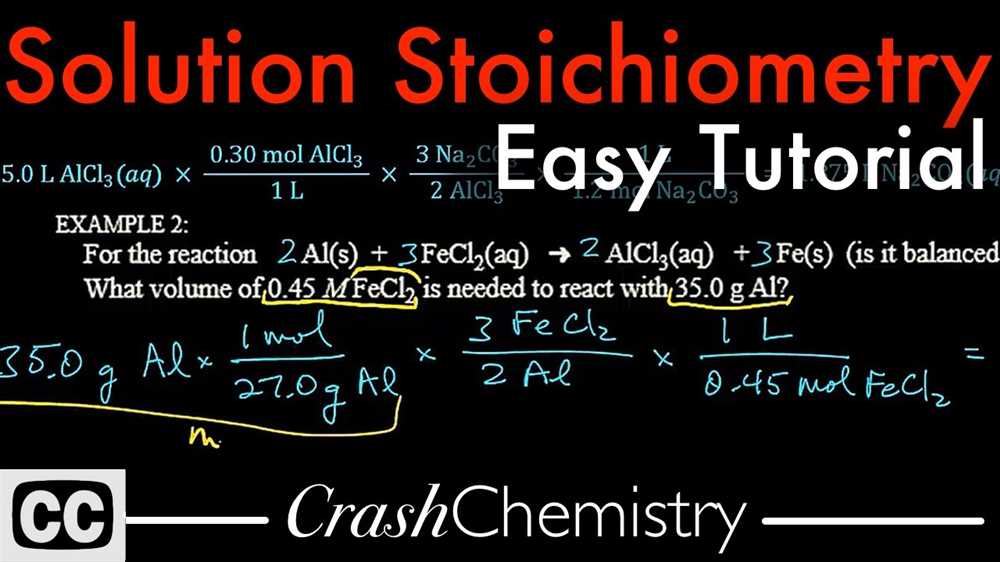
Dimensional analysis is a powerful tool in stoichiometry. By setting up conversion factors and canceling units, you can ensure that your calculations are accurate and that the units of your final answer are correct. Mastering dimensional analysis will greatly simplify stoichiometric calculations.
4. Take note of stoichiometric ratios
Stoichiometric ratios, also known as mole ratios, are the coefficients in a balanced chemical equation. These ratios provide the relationship between the number of moles of reactants and products involved in a reaction. Pay close attention to the stoichiometric ratios when setting up your calculations.
5. Break down complex stoichiometry problems into smaller steps
Complex stoichiometry problems can seem overwhelming at first. However, breaking them down into smaller steps can make them more manageable. Take the time to carefully read and analyze the problem, identify the given information, and plan your approach before diving into the calculations.
6. Practice, practice, practice
As with any skill, practice is key to mastering stoichiometry. Work through a variety of stoichiometry problems to build your understanding and improve your problem-solving abilities. The more you practice, the more comfortable and confident you will become in applying stoichiometry concepts to different scenarios.
By applying these tips and strategies, you can enhance your proficiency in stoichiometry and tackle even the most challenging problems with ease. Remember to approach each problem with a clear understanding of the concepts and take the time to analyze and plan your calculations. With practice and perseverance, you will become a stoichiometry expert.