
Stoichiometry, often called the language of chemistry, is a crucial skill for any student studying the subject. It involves using the principles of balanced chemical equations to calculate the quantities of reactants and products involved in a chemical reaction. To master this skill, students need plenty of practice, and a stoichiometry practice worksheet is the perfect tool for reinforcing their understanding.
However, without an answer key, students may find it challenging to assess their progress and identify any mistakes in their calculations. This is where a stoichiometry practice worksheet answer key becomes invaluable. With clear and concise answers provided, students can compare their solutions and learn from any errors they may have made.
The answer key serves as a gauge for students to measure their proficiency in stoichiometry. It not only provides the correct answers but also offers explanations and step-by-step calculations. This helps students understand the underlying concepts and strategies involved in solving stoichiometry problems.
With a stoichiometry practice worksheet answer key in hand, students can gain confidence in their ability to perform stoichiometric calculations accurately. It reinforces their understanding of the stoichiometry principles while allowing them to practice and improve their problem-solving skills. By continuously working through practice problems with the answer key, students can master the art of chemical calculations and excel in their chemistry studies.
Stoichiometry Practice Worksheet Answer Key
Stoichiometry is an essential concept in chemistry that involves the calculation of quantities in a chemical reaction. It helps determine the amount of reactants required to produce a certain amount of product and vice versa. To practice stoichiometry, students are often given worksheets with various chemical equations and asked to solve for specific quantities.
The answer key for a stoichiometry practice worksheet provides the correct solutions to the given problems. It serves as a reference for students to check their work and ensure accuracy in their calculations. The answer key typically includes the balanced chemical equation, the molar ratios, and the calculated quantities of reactants and products.
Using the answer key, students can compare their answers and identify any errors or misconceptions they may have had. It allows them to understand the steps involved in solving stoichiometry problems and reinforces their understanding of the concepts involved. The answer key also provides a way for instructors to assess students’ progress and provide feedback.
Example Answer Key:
| Chemical Equation | Molar Ratios | Given Quantity | Calculated Quantity |
|---|---|---|---|
| 2H2 + O2 → 2H2O | 2 moles H2 : 1 mole O2 | 4 moles H2 | 2 moles O2 |
| 2C2H6 + 7O2 → 4CO2 + 6H2O | 2 moles C2H6 : 7 moles O2 | 3 moles C2H6 | 10.5 moles O2 |
| Fe + HCl → FeCl3 + H2 | 1 mole Fe : 3 moles HCl | 7 moles HCl | 2.33 moles Fe |
By using the stoichiometry practice worksheet answer key, students can reinforce their understanding of stoichiometry and improve their problem-solving skills in chemistry.
What is stoichiometry?
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows us to calculate the amounts of substances involved in a chemical reaction, based on the balanced chemical equation.
In stoichiometry, we use the mole concept to relate the amounts of substances. A mole is a unit of measurement in chemistry, representing a specific number of atoms, molecules, ions, or other particles. It allows us to compare the amounts of different substances in a balanced chemical equation.
The key concept in stoichiometry is the mole ratio, which is derived from the coefficients of the balanced chemical equation. The mole ratio represents the relative amounts of reactants and products in a chemical reaction. It allows us to convert between the amounts of different substances involved in the reaction.
To solve stoichiometry problems, we usually start with the given amount of one substance and use the mole ratio to calculate the amount of another substance. This involves converting the given amount to moles, using the mole ratio to determine the moles of the desired substance, and then converting back to the desired units.
Stoichiometry is essential in various areas of chemistry, including analyzing and predicting the outcomes of chemical reactions, determining the theoretical yield and percent yield of a reaction, and understanding the relationships between reactants and products.
In conclusion, stoichiometry is a fundamental concept in chemistry that allows us to quantitatively analyze and understand chemical reactions. It provides us with the tools to calculate the amounts of substances involved in a reaction and predict the outcomes of chemical processes.
Why is stoichiometry important in chemistry?
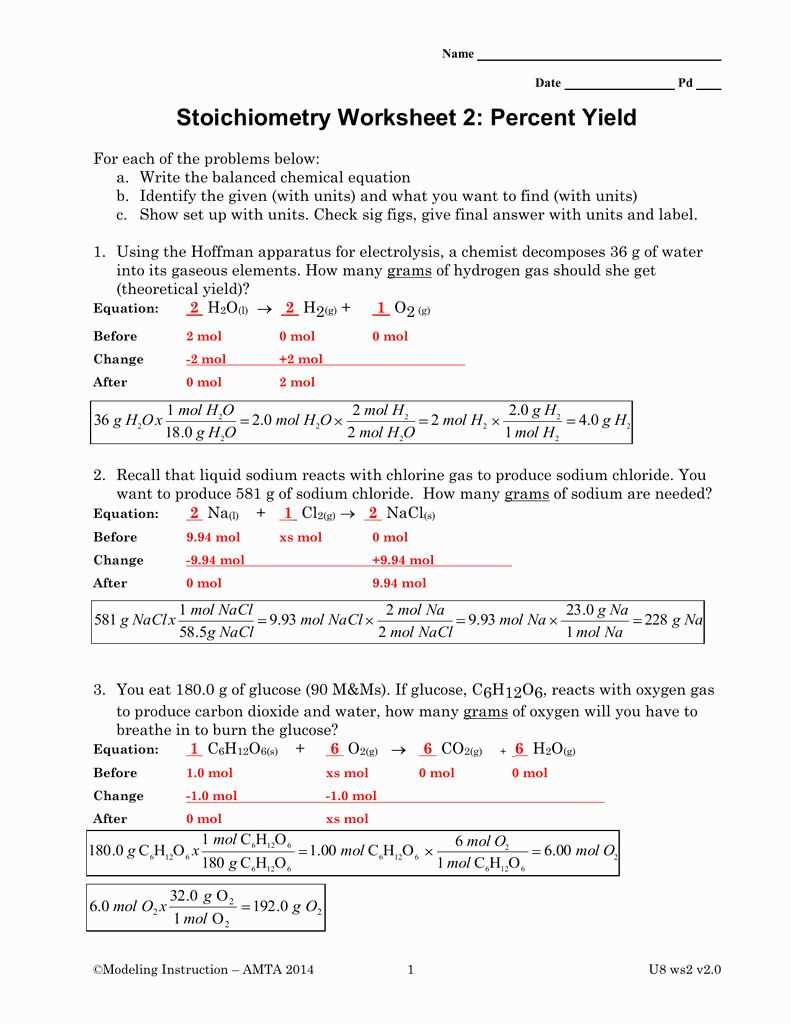
Stoichiometry is a fundamental concept in chemistry that plays a crucial role in understanding and predicting chemical reactions. It provides a quantitative understanding of the relationships between reactants and products in a chemical reaction. By using stoichiometry, chemists can determine the amount of substances involved in a reaction, predict the amount of product that will be formed, and analyze the efficiency of a reaction.
One of the main reasons why stoichiometry is important in chemistry is that it allows chemists to calculate the amount of reactants needed to produce a desired amount of product. This is essential for industries that rely on chemical reactions, such as pharmaceuticals, manufacturing, and environmental science. Stoichiometry helps to optimize reactions by ensuring that the correct amounts of reactants are used, minimizing waste and maximizing efficiency.
Furthermore, stoichiometry is essential for understanding the theoretical and practical aspects of chemical reactions. It enables chemists to balance chemical equations, which is a necessary step in analyzing reactions and determining the stoichiometric ratios between reactants and products. This information can then be used to calculate the theoretical yield of a reaction, which is the maximum amount of product that can be obtained.
Overall, stoichiometry is a fundamental concept in chemistry that is important for various applications. It provides the basis for understanding chemical reactions, predicting the amounts of substances involved, and optimizing reaction conditions. By applying stoichiometry, chemists can make informed decisions and advancements in fields such as medicine, industry, and environmental science.
How to balance chemical equations?

When working with chemical reactions, it is important to make sure that the equation is balanced. A balanced chemical equation shows that there is an equal number of atoms on both sides of the equation. This is important because it follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
To balance a chemical equation, there are a few steps that can be followed:
- Identify the reactants and products: Determine the substances involved in the reaction and write them down. Reactants are the substances that are present before the reaction takes place, while products are the substances that are formed as a result of the reaction.
- Count the number of atoms on each side: Look at the chemical formulas of the reactants and products and count the number of atoms of each element on both sides of the equation.
- Start by balancing the elements that appear in only one reactant and one product: Begin by balancing the elements that are present in only one reactant and one product. Adjust the coefficients in front of each formula to ensure that there is an equal number of atoms of each element on both sides.
- Balance the remaining elements: After balancing the elements that appear in only one reactant and one product, move on to the elements that appear in multiple reactants or products. Adjust the coefficients as needed to ensure that the number of atoms of each element is equal on both sides.
- Check and simplify: Once all the elements are balanced, double-check to ensure that the number of atoms of each element is the same on both sides. If necessary, simplify the equation by dividing all coefficients by their greatest common divisor.
By following these steps, it is possible to balance a chemical equation and ensure that all the atoms are accounted for. This is an important skill in chemistry and allows for a better understanding of how reactions occur.
What is the mole ratio in stoichiometry?
In stoichiometry, the mole ratio refers to the ratio of moles of one substance to moles of another substance in a balanced chemical equation. It is an important concept used to calculate the quantities of reactants and products involved in a chemical reaction.
To determine the mole ratio, one must first examine the balanced chemical equation for the reaction. The coefficients of the balanced equation represent the number of moles of each substance involved in the reaction. These coefficients can be used to establish the mole ratio between the reactants and products.
The mole ratio is expressed as a fraction or ratio, with the number of moles of one substance in the numerator and the number of moles of another substance in the denominator. This ratio allows chemists to determine the relative amounts of substances that will react or be produced in a given reaction.
For example, in the chemical equation 2H₂ + O₂ → 2H₂O, the mole ratio of H₂ to O₂ is 2:1. This means that for every 2 moles of H₂ used in the reaction, 1 mole of O₂ will be consumed. Similarly, for every 2 moles of H₂O produced, 1 mole of O₂ is required.
The mole ratio is a fundamental concept in stoichiometry and is used to perform calculations such as determining the limiting reactant, predicting the amount of product formed, and calculating the percent yield of a reaction.
How to calculate the mass of products in a chemical reaction?

Calculating the mass of products in a chemical reaction is an essential part of stoichiometry, which is the study of the quantitative relationships between reactants and products in a chemical reaction. By using the balanced chemical equation, we can determine the mole ratio between the reactants and products, and then convert the moles of the given reactant to the mass of the desired product.
To calculate the mass of products, we first need to write a balanced chemical equation for the reaction. This equation tells us the molar ratios between the reactants and products. Once we have the balanced equation, we can determine the mole ratio between the desired product and the given reactant.
Next, we need to convert the moles of the given reactant to the moles of the desired product using the mole ratio. To do this, we multiply the moles of the given reactant by the mole ratio of the desired product to the given reactant. The mole ratio is based on the coefficients in the balanced chemical equation.
Finally, we can convert the moles of the desired product to its mass by using the molar mass of the product. The molar mass is the mass of one mole of a substance and is expressed in grams per mole. We multiply the moles of the desired product by its molar mass to get the mass of the product.
In summary, to calculate the mass of products in a chemical reaction, we need to use the balanced chemical equation to determine the mole ratio between the reactants and products. Then, we convert the moles of the given reactant to the moles of the desired product using the mole ratio. Finally, we multiply the moles of the desired product by its molar mass to obtain the mass of the product.
How to Calculate the Number of Moles in a Chemical Reaction?
In chemistry, stoichiometry is the study of the numerical relationships between the amounts of reactants and products in a chemical reaction. One of the fundamental calculations in stoichiometry is determining the number of moles involved in a chemical reaction. Moles are a unit of measurement used to quantify the amount of a substance in a reaction.
To calculate the number of moles in a chemical reaction, there are a few steps you can follow:
- Write the balanced chemical equation: Start by writing the balanced chemical equation for the reaction. This equation shows the reactants and products involved and their respective coefficients, which represent the number of moles of each substance.
- Identify the known and unknown quantities: Determine which quantities are known and which are unknown. You may be given the mass of a substance or the volume of a gas, which can be used to calculate the number of moles.
- Convert the known quantity to moles: Use the molar mass of the substance to convert the known quantity to moles. The molar mass is the mass of one mole of a substance and is expressed in grams per mole. This conversion involves dividing the given mass or volume by the molar mass.
- Use the stoichiometric coefficients: Refer to the balanced equation to determine the stoichiometric coefficients of the substances involved. These coefficients represent the ratio of moles between reactants and products. Use these ratios to calculate the number of moles of the unknown substance.
- Calculate the number of moles: Apply the stoichiometric ratios to calculate the number of moles of the unknown substance. This is done by multiplying the moles of the known substance by the ratio of moles of the unknown substance to the moles of the known substance.
By following these steps, you can accurately calculate the number of moles involved in a chemical reaction. Stoichiometry calculations are crucial for understanding the relationships between reactants and products and predicting the outcome of chemical reactions.
What is the difference between theoretical and actual yield?
Theoretical and actual yield are two important concepts in the field of stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions. Theoretical yield refers to the maximum amount of product that can be obtained from a given amount of reactants, based on the stoichiometry of the reaction and assuming complete conversion of reactants into products without any loss.
Actual yield, on the other hand, refers to the amount of product that is actually obtained from a reaction in a laboratory or industrial setting. It takes into account various factors that may affect the yield, such as incomplete conversion of reactants, side reactions, impurities, and experimental limitations. Actual yield is often lower than the theoretical yield due to these factors, and it can be influenced by a range of external conditions, such as temperature, pressure, and the presence of catalysts.
To calculate the percent yield, which is a measure of the efficiency of a reaction, one compares the actual yield to the theoretical yield. The percent yield is calculated using the formula: percent yield = (actual yield / theoretical yield) x 100%. A percent yield of 100% indicates that the actual yield is equal to the theoretical yield, while a percent yield lower than 100% indicates that the actual yield is lower than the theoretical yield.
- In summary, theoretical yield is the maximum amount of product that can be obtained based on the stoichiometry of the reaction, while actual yield is the amount of product obtained in practice.
- Actual yield is often lower than theoretical yield due to factors such as incomplete conversion and side reactions.
- The percent yield is a measure of the efficiency of a reaction and is calculated by comparing the actual yield to the theoretical yield.