When studying chemistry and learning about solutions, one important concept to understand is solubility. Solubility refers to the ability of a substance to dissolve in another substance.
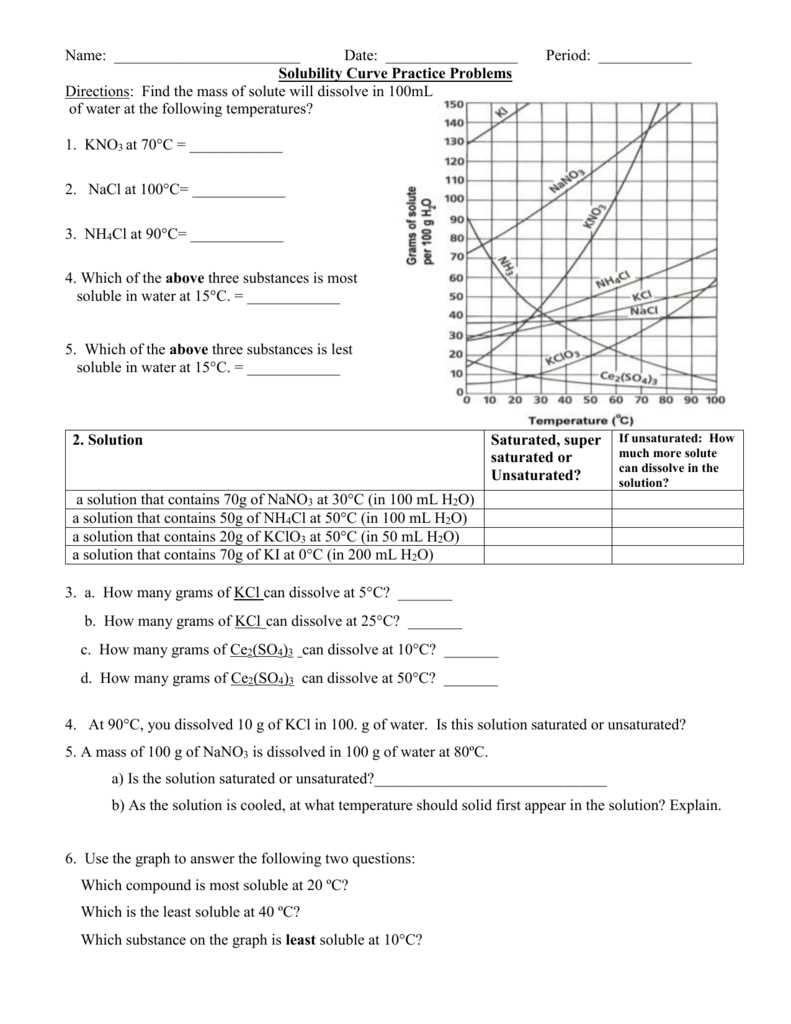
A solubility curve is a graph that shows the amount of solute that can be dissolved in a given amount of solvent at a specific temperature. It provides valuable information about the solubility of different substances under different conditions.
In the Unit 12 Solutions Solubility Curves Worksheet, students are presented with various solubility curves and are asked to interpret the data and answer questions about the solubility of specific substances. This worksheet helps students develop their understanding of solubility and how it is affected by factors such as temperature and concentration.
The answer key for the Unit 12 Solutions Solubility Curves Worksheet provides students with the correct answers to the questions posed in the worksheet. It allows students to check their work and ensure they have a solid understanding of the material.
Unit 12 Solutions Solubility Curves Worksheet Answer Key
In the subject of chemistry, understanding solubility curves is crucial. A solubility curve is a graphical representation of the solubility of a solute in a solvent at various temperatures. In Unit 12, students were given a solubility curves worksheet and now, we will provide the answer key to help them check their answers and further comprehend the topic.
Answer Key:
- Question 1: Based on the solubility curve, what is the solubility of Substance X at 20°C?
- Answer: 40g/100mL
- Question 2: At which temperature does the solubility of Substance Y decrease?
- Answer: 50°C
- Question 3: What is the maximum amount of Substance Z that can be dissolved in 200 mL of water at 30°C?
- Answer: 80g
- Question 4: Which substance is least soluble in water at 40°C?
- Answer: Substance A
- Question 5: True or False: The solubility of Substance B increases with an increase in temperature.
- Answer: True
By using this answer key, students can determine whether they have correctly interpreted the solubility curve and understand the relationship between temperature and solubility. It is essential for them to grasp this concept as it has practical applications in various fields such as pharmaceuticals, environmental science, and materials science.
What Are Solutions?
A solution is a homogeneous mixture made up of two or more substances. In a solution, the solute is the substance that is dissolved, and the solvent is the substance that does the dissolving. The solute is typically present in a smaller amount compared to the solvent. Solutions can be found in various states, such as solid, liquid, or gas.
Solutions are formed through a process called solvation, where the solvent molecules surround the solute particles and separate them, allowing them to mix evenly. This process occurs due to the attractive forces between the solute and solvent particles. The resulting solution has uniform properties throughout, which means that it has the same composition and properties in every part of the mixture.
One way to classify solutions is based on their solubility, which refers to how well a solute can dissolve in a particular solvent. Solubility is often represented using solubility curves, which show the maximum amount of solute that can dissolve in a given amount of solvent at different temperatures. These solubility curves can be used to determine whether a solution is saturated, unsaturated, or supersaturated.
Understanding Solubility Curves
The solubility of a substance refers to its ability to dissolve in a particular solvent. Solubility curves are graphical representations that show the relationships between the solubility of a substance and factors such as temperature and pressure. These curves provide valuable information about how the solubility of a substance changes under different conditions, which is important in various fields of science, including chemistry, biology, and environmental science.
When examining a solubility curve, it is important to consider the units used for both the solute and solvent. Typically, solubility is given in grams of solute per 100 grams of solvent, but it can also be expressed in other units such as moles or volume. The curve itself is usually plotted using temperature on the x-axis and solubility on the y-axis. The shape of the curve can vary depending on the substance being dissolved, with some substances showing a gradual increase in solubility with temperature (positive slope), while others may exhibit a decrease in solubility with temperature (negative slope).
One key concept that can be understood from solubility curves is the saturation point. The saturation point represents the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. Any additional solute added beyond this point will not dissolve and will form a precipitate. By analyzing the solubility curve, scientists can determine the saturation point for a particular substance and temperature, which can be used in various applications such as manufacturing processes or pharmaceutical formulations.
Additionally, solubility curves can also be used to predict the behavior of a substance when the temperature changes. For example, if the solubility of a substance increases with temperature, it suggests that the substance is an endothermic compound, as it requires heat to break the intermolecular forces and dissolve. On the other hand, if the solubility decreases with temperature, it indicates that the substance is exothermic, as it releases heat when dissolving.
In conclusion, solubility curves provide a valuable tool for understanding the solubility of substances under different conditions. By analyzing these curves, scientists can determine the saturation point, predict the behavior of substances, and make informed decisions in various scientific and industrial applications.
Factors Affecting Solubility
Solubility refers to the ability of a substance to dissolve in a solvent and form a homogeneous mixture. Several factors can affect the solubility of a solute, including temperature, pressure, and the nature of the solute and solvent.
Temperature: One of the main factors that affects solubility is temperature. In general, solubility increases with an increase in temperature for solid solutes in liquid solvents. This is because higher temperatures provide more energy for the solvent particles to collide with and break apart the solute particles. However, for some solutes, such as certain gases, solubility decreases with an increase in temperature.
Pressure: Pressure also plays a role in determining solubility, particularly for gases. According to Henry’s law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. As the pressure increases, the solubility of the gas in the liquid also increases.
Nature of the Solute and Solvent: The chemical nature of the solute and solvent can greatly impact their solubility. Polar solutes, such as salts, sugars, and alcohols, tend to be more soluble in polar solvents like water. On the other hand, nonpolar solutes, such as hydrocarbons, are more soluble in nonpolar solvents like oil. Additionally, the interactions between the solute and solvent molecules, such as hydrogen bonding or dipole-dipole interactions, can affect solubility.
Understanding these factors is crucial for predicting and controlling solubility in various chemical processes, such as pharmaceutical manufacturing, environmental remediation, and chemical synthesis.
How to Read a Solubility Curve
Solubility curves are graphical representations of the relationship between solubility (in g/100 mL) and temperature (in °C) for a specific substance. They provide valuable information about how much of a substance can dissolve in a given amount of solvent at different temperatures. To properly read a solubility curve, it is important to understand the key components and how they are represented.
The x-axis of a solubility curve represents temperature in degrees Celsius, while the y-axis represents solubility in grams of solute per 100 milliliters of solvent. The solubility curve itself is a line or curve that connects specific points, each of which represents the maximum amount of solute that can dissolve at a given temperature.
Here are some key tips to keep in mind when reading a solubility curve:
- Increasing temperature: As you move from left to right along the x-axis (increasing temperature), the solubility of the substance generally increases. This means that more solute can dissolve in the solvent at higher temperatures.
- Plateaus or curves: A solubility curve may have plateaus or curves, indicating a change in the relationship between temperature and solubility. Plateaus suggest that solubility remains constant within a specific temperature range, while curves indicate that solubility changes at different rates within the curve.
- Saturation point: The point at which a solute can no longer dissolve in the solvent, even with increased temperature, is known as the saturation point. This is typically represented by a horizontal line on the solubility curve.
- Interpolation: When you need to determine the solubility at a temperature between the given points on the solubility curve, you can use interpolation. This involves estimating the solubility based on the shape of the curve and the given data points.
- Extrapolation: In some cases, you may need to estimate the solubility at a temperature outside the given range. This is called extrapolation and involves extending the solubility curve beyond the known data points. However, extrapolation can be less accurate and should be used with caution.
Overall, reading a solubility curve requires an understanding of how temperature affects solubility and how to interpret the graphical representation of the data. By following the key tips outlined above, you can effectively analyze a solubility curve and extract valuable information about the solubility of a substance at different temperatures.
Interpreting the Worksheet
The worksheet on Unit 12 solutions solubility curves provides students with a series of questions and tasks that help them understand and interpret solubility curves. Solubility curves depict the relationship between the solubility of a substance and temperature, allowing us to predict how much of a solute will dissolve in a given solvent at various temperatures.
The worksheet begins with a series of solubility curve graphs, each depicting the solubility of a different substance. Students are asked to analyze the graphs, identify the solute and solvent, and use the information to answer questions about the solubility of the substance at different temperatures. This helps students develop a clear understanding of how solubility changes with temperature.
The worksheet also includes a section on interpreting solubility curves. Students are given a solubility curve graph and asked to determine the temperature at which a certain amount of solute will dissolve, or the amount of solute that will dissolve at a given temperature. This requires students to carefully read and interpret the graph, as well as apply their knowledge of solubility curves.
In addition, the worksheet provides practice problems where students are given a solubility curve graph and asked to calculate the solubility of a substance at a specific temperature. This helps students develop their skills in reading and interpreting solubility curves, as well as applying mathematical calculations to solve problems.
Overall, the Unit 12 solutions solubility curves worksheet is a valuable tool for students to practice and develop their understanding of solubility curves. It provides them with the opportunity to analyze and interpret solubility curve graphs, apply their knowledge to solve problems, and reinforce their understanding of how temperature affects solubility.
Answer Key for Solubility Curves Worksheet
In the study of chemistry, understanding solubility is essential. Solubility curves help us determine the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature. Solubility curves are represented as graphs, and this worksheet provides a series of questions based on these graphs.
The answer key for the Solubility Curves Worksheet is designed to provide correct answers and explanations for each question. It allows students to check their responses and understand any mistakes they may have made. By using the answer key, students can gain a better understanding of solubility curves and improve their knowledge of the subject.
The answer key includes a detailed explanation for each question, pointing out the key factors that determine solubility. It covers topics such as the effect of temperature on solubility, understanding concentration curves, and calculating the maximum solute concentration in a solution. The explanations provide step-by-step guidance, making it easier for students to grasp the concepts.
By using the answer key, students can confidently evaluate their progress and identify areas where they need further practice or clarification. It serves as a valuable study tool and allows students to strengthen their understanding of solubility concepts.
Example Question:
Question: At what temperature is the solubility of potassium nitrate (KNO3) approximately 120 g/100 g of water?
Answer: The solubility of potassium nitrate at the given temperature can be determined by following the vertical line on the graph that intersects with the solubility curve of KNO3. In this case, the approximate temperature is 70°C.
Using the answer key for the Solubility Curves Worksheet, students can enhance their understanding of solubility and further develop their chemistry skills.
Common Mistakes and Troubleshooting
When working with solubility curves and completing the Unit 12 solutions solubility curves worksheet, there are several common mistakes that students often make. This section will address these mistakes and provide troubleshooting tips to help students avoid them.
1. Misreading the solubility curve
One common mistake is misreading the solubility curve. This can happen if the graph is not properly labeled or if students do not fully understand how to interpret the curve. To avoid this mistake, it is important to carefully read the instructions and review the graph before attempting to answer the questions. Pay attention to the axes and units.
2. Incorrectly identifying the solubility of a substance
Another mistake students often make is incorrectly identifying the solubility of a substance based on the solubility curve. This can happen if students do not accurately read the point on the curve or if they do not understand how solubility is determined. To troubleshoot this issue, double-check the coordinates of the point on the curve and review the definitions of solubility.
3. Failing to use the correct units
It is important to use the correct units when working with solubility curves. Students may forget to include units or use the wrong units, which can lead to incorrect answers. To avoid this mistake, always include the appropriate units when writing your responses and double-check your work before submitting.
4. Not considering factors that affect solubility
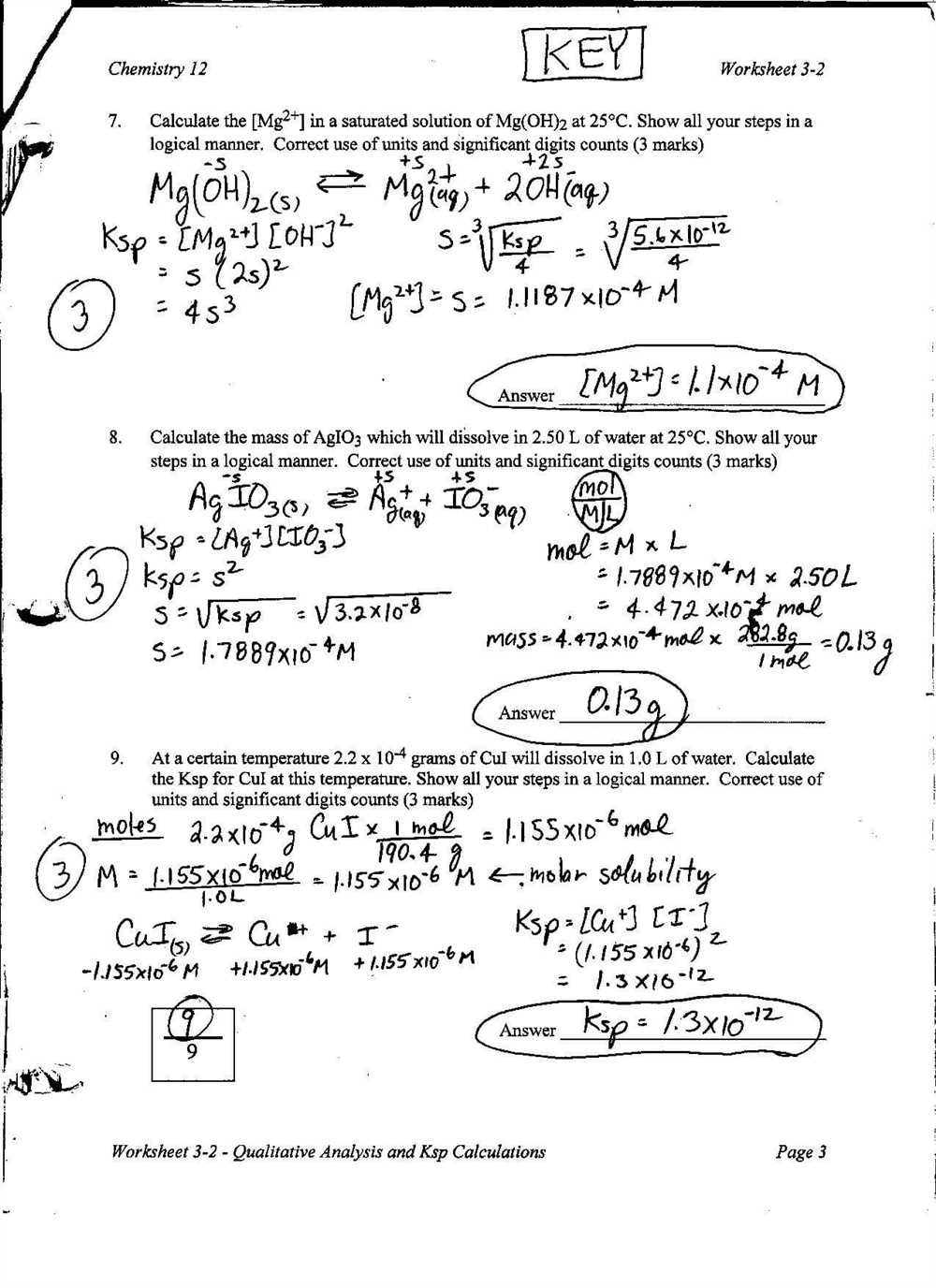
When answering questions about solubility using the solubility curve, it is important to consider factors that can affect solubility, such as temperature. Failing to take these factors into account can lead to incorrect conclusions. Make sure to carefully read the question and consider all relevant factors before providing your answer.
By being aware of these common mistakes and using the troubleshooting tips provided, students can improve their understanding of solubility curves and successfully complete the Unit 12 solutions solubility curves worksheet. Practice and attention to detail are key to mastering this topic.