Chemistry is a fascinating field of study that explores the properties and interactions of matter. Unit 2 Worksheet 1 in chemistry often includes questions related to chemical reactions, balancing equations, and stoichiometry. These worksheets provide students with an opportunity to apply their knowledge and problem-solving skills to real-world scenarios.
One important topic covered in Unit 2 Worksheet 1 is the concept of chemical reactions. Students are tasked with identifying and balancing chemical equations, understanding the reactants and products involved, and predicting the outcome of a reaction based on the given information. This helps students develop a deeper understanding of how molecules interact and combine to form new substances.
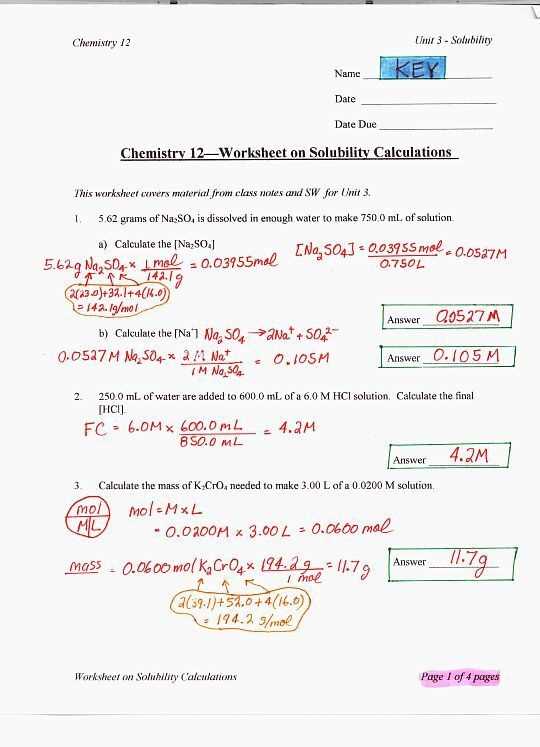
Another key concept covered in these worksheets is stoichiometry, which involves using balanced chemical equations to determine the quantitative relationships between reactants and products. Students learn to calculate the amount of reactant needed or the amount of product formed in a reaction, as well as understand the concept of mole ratio and molar mass.
Overall, Unit 2 Worksheet 1 in chemistry is an essential tool for students to reinforce their understanding of chemical reactions, balancing equations, and stoichiometry. It allows them to apply their knowledge in practical scenarios and build a solid foundation for further study in the field.
Unit 2 Worksheet 1 Chemistry Answers: Exploring the Basics of Chemistry
Chemistry is the scientific study of matter and the changes it undergoes. In Unit 2 Worksheet 1, we delve into the fundamentals of chemistry and explore various concepts and principles related to this field. This worksheet allows students to grasp the basics and lay a solid foundation for further learning in chemistry.
One of the key topics covered in this worksheet is the structure of atoms. Atoms are the building blocks of matter and understanding their composition is essential in chemistry. Students are introduced to the concept of atomic number, mass number, and isotopes. They learn how to calculate the number of protons, neutrons, and electrons in an atom using the periodic table and the atomic symbols of elements.
Another important aspect covered in this worksheet is the periodic table. The periodic table is a tabular arrangement of elements based on their atomic numbers. Students learn how to read and interpret the periodic table, including the information provided for each element such as its atomic symbol, atomic number, and atomic mass. They also learn about the organization of elements into groups and periods, and the trend of properties across the periodic table.
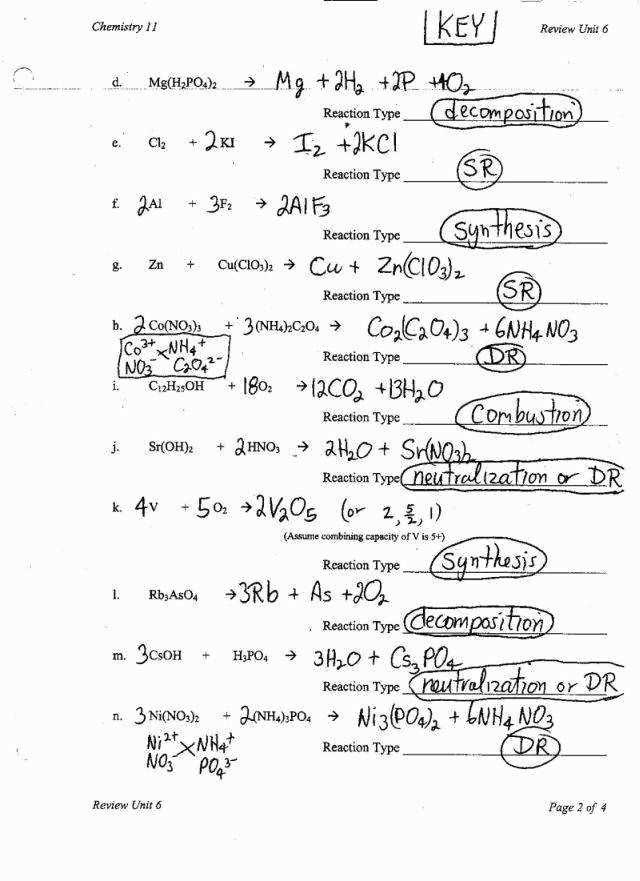
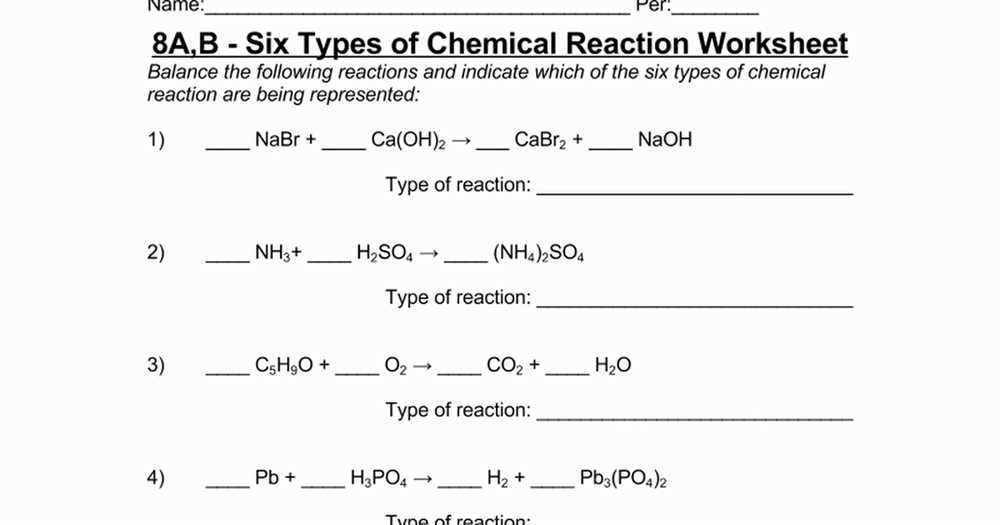
In addition, this worksheet delves into chemical equations and reactions. Students learn how to balance chemical equations by ensuring that the number of atoms of each element is the same on both sides of the equation. They are introduced to different types of reactions, such as synthesis, decomposition, combustion, and displacement reactions, and learn how to identify them based on the reactants and products involved.
Overall, Unit 2 Worksheet 1 provides a solid introduction to the basics of chemistry. It equips students with the necessary knowledge and skills to understand and analyze various chemical phenomena and lays the groundwork for further exploration of this fascinating scientific field.
What is Chemistry and Why is it Important?
Chemistry is the scientific study of matter, its properties, and the changes it undergoes. It is often considered the central science because it connects and bridges the physical, biological, and Earth sciences. Understanding chemistry is crucial in many aspects of everyday life and plays a significant role in various industries such as medicine, agriculture, and technology.
In medicine, chemistry is vital for drug discovery and development. Pharmaceutical chemists study the chemical structures and properties of substances to create new drugs that can treat diseases effectively. They also analyze the interactions between drugs and the human body to determine the appropriate dosage and potential side effects. Without the advancements made in chemistry, many life-saving medications would not exist.
Chemistry also plays a crucial role in agriculture and food production. Agricultural chemists develop fertilizers that enhance crop growth and yield, ensuring an abundant and nutritious food supply. They also study pesticides and herbicides to protect crops from pests and weeds. Furthermore, chemists analyze the composition of food products to ensure their safety and quality.
Moreover, chemistry has revolutionized technology and has contributed to the development of various materials and products. Materials scientists and chemists work together to create new materials with specific properties, such as plastics, polymers, and alloys. They also study catalysts, which are essential in various industrial processes, including manufacturing, energy production, and pollution control. The advancements in chemistry have led to the creation of modern technologies that have transformed our lives, such as smartphones, solar panels, and electric vehicles.
In conclusion, chemistry is a fundamental science that helps us understand the world around us and enables technological advancements. It plays a crucial role in various sectors, including medicine, agriculture, and technology. Without chemistry, many of the advancements and innovations that have improved our lives would not be possible. It is an essential field of study that continues to shape and revolutionize our society.
Understanding the Unit 2 Worksheet 1 Chemistry Questions
In Unit 2 Worksheet 1 of chemistry, we are presented with a series of questions that aim to test our understanding of key concepts in the field. These questions cover various topics such as atomic structure, the periodic table, and chemical reactions. It is important to carefully read and analyze each question in order to provide accurate and concise answers.
One of the questions in Unit 2 Worksheet 1 asks us to identify the number of protons, neutrons, and electrons in a given element. To answer this question, we need to know the atomic number, which represents the number of protons, and the atomic mass, which represents the combined number of protons and neutrons. By subtracting the atomic number from the atomic mass, we can determine the number of neutrons. The number of electrons is equal to the number of protons in a neutral atom.
Another question in the worksheet asks us to determine the number of valence electrons in a specific element. Valence electrons are the electrons located in the outermost energy level of an atom, and they play a crucial role in determining an element’s chemical properties. To find the number of valence electrons, we need to look at the element’s group number on the periodic table. Elements in the same group have the same number of valence electrons.
Furthermore, the worksheet includes questions related to chemical reactions, such as balancing chemical equations. Balancing chemical equations involves adjusting the coefficients in the equation to ensure that the number of atoms of each element is equal on both sides of the equation. This is done by applying the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Overall, Unit 2 Worksheet 1 in chemistry provides an opportunity to practice and reinforce our understanding of important concepts in the field. By carefully analyzing the questions and applying our knowledge of atomic structure, the periodic table, and chemical reactions, we can successfully answer the questions and further enhance our understanding of chemistry as a whole.
Key Concepts in Unit 2 Worksheet 1 Chemistry
In Unit 2 Worksheet 1 of Chemistry, we cover several key concepts related to the study of matter and its properties. These concepts are essential for understanding the basic principles of chemistry and form the foundation for more advanced topics.
1. Matter and Its States
One of the fundamental concepts in chemistry is the study of matter and its states. Matter refers to anything that has mass and occupies space, and it can exist in three main states: solid, liquid, and gas. Each state has distinct properties and behaviors, and understanding these states is crucial for analyzing chemical reactions and transformations.
2. Physical and Chemical Changes
Unit 2 Worksheet 1 also introduces the differentiation between physical and chemical changes. Physical changes involve alterations in the state or properties of matter without any change in its chemical composition. Examples include melting, boiling, and dissolving. On the other hand, chemical changes result in the formation of new substances with different chemical composition and properties. Examples include burning, rusting, and fermentation.
3. Elements and Compounds
The concept of elements and compounds is another important topic covered in Unit 2 Worksheet 1. Elements are pure substances that cannot be further broken down into simpler substances by ordinary chemical means. Compounds, on the other hand, are substances composed of two or more elements chemically combined in fixed ratios. Understanding the difference between elements and compounds is essential for studying the composition and behavior of matter.
4. Mixtures and Solutions
Lastly, Unit 2 Worksheet 1 explores the concept of mixtures and solutions. Mixtures are combinations of two or more pure substances that are physically combined but not chemically bonded. They can be further classified into homogeneous and heterogeneous mixtures. Solutions, on the other hand, are homogeneous mixtures in which one substance (the solute) is dissolved in another substance (the solvent) at the molecular level. Understanding mixtures and solutions is crucial for analyzing the properties and behavior of various substances in various contexts.
In conclusion, Unit 2 Worksheet 1 of Chemistry introduces several key concepts that form the foundation for understanding the basic principles of chemistry. These concepts include matter and its states, physical and chemical changes, elements and compounds, as well as mixtures and solutions. Mastering these concepts is essential for building a strong understanding of the field of chemistry and its applications.
Atomic Structure and the Periodic Table
Atoms are the building blocks of all matter. They are composed of subatomic particles, including protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. The number of protons in an atom determines its atomic number, which defines the element. For example, hydrogen has one proton, so its atomic number is 1.
The periodic table is a tabular arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. It is organized into rows called periods and columns called groups. Each element is represented by a symbol and is classified into different categories, such as metals, non-metals, and metalloids. The periodic table provides important information about an element’s properties, such as its atomic mass, valence electrons, and the number of energy levels.
Some key concepts related to atomic structure and the periodic table:
- Isotopes: Atoms of the same element can have different numbers of neutrons, resulting in isotopes with different atomic masses.
- Electron Configuration: The arrangement of electrons in the energy levels of an atom. It determines an element’s chemical behavior.
- Valence Electrons: Electrons in the outermost energy level of an atom. They are involved in chemical bonding and determine the element’s reactivity.
- Periodic Trends: Patterns observed in the properties of elements as you move across a period or down a group in the periodic table. Examples include atomic radius, ionization energy, and electronegativity.
In summary, understanding atomic structure and the periodic table is essential for understanding the properties and behaviors of elements. It allows scientists to predict chemical reactions, discover new elements, and develop new materials with specific properties.
Chemical Bonds and Reactions
A chemical bond is the force that holds atoms together in a compound. There are three types of chemical bonds: ionic bonds, covalent bonds, and metallic bonds. Ionic bonds occur between a metal and a nonmetal, where one atom gives up an electron to another atom. Covalent bonds occur between two nonmetals, where atoms share electrons. Metallic bonds occur between metal atoms, where electrons detach from their parent atoms and form a “sea” of mobile electrons.
In a chemical reaction, atoms are rearranged to form new substances. There are several types of chemical reactions, including synthesis reactions, decomposition reactions, single replacement reactions, and double replacement reactions. In a synthesis reaction, two or more substances combine to form a single product. In a decomposition reaction, a single compound breaks down into two or more simpler substances. In a single replacement reaction, one element replaces another element in a compound. In a double replacement reaction, the positive ions of two compounds switch places.
Examples:
- In an ionic bond, sodium (Na) and chlorine (Cl) combine to form sodium chloride (NaCl).
- In a covalent bond, hydrogen (H) and oxygen (O) combine to form water (H2O).
- In a metallic bond, atoms of copper (Cu) form a “sea” of mobile electrons.
- In a synthesis reaction, hydrogen (H2) and oxygen (O2) combine to form water (H2O).
- In a decomposition reaction, water (H2O) breaks down into hydrogen (H2) and oxygen (O2).
- In a single replacement reaction, zinc (Zn) replaces hydrogen (H) in hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen gas (H2).
- In a double replacement reaction, silver nitrate (AgNO3) and sodium chloride (NaCl) combine to form silver chloride (AgCl) and sodium nitrate (NaNO3).
Chemical bonds and reactions play a fundamental role in understanding how substances interact and transform in the world of chemistry.
Step-by-Step Solutions to Unit 2 Worksheet 1 Chemistry
In Unit 2 Worksheet 1 of Chemistry, students are presented with a series of questions and problems that require them to apply their knowledge of different chemical concepts. These concepts include atomic structure, ions, isotopes, and electron configuration. To successfully solve this worksheet, students need to understand the basics of these concepts and be able to apply them in various scenarios.
One question in the worksheet asks students to identify the number of protons, neutrons, and electrons in different isotopes of an element. To solve this, students must first determine the atomic number of the element, which represents the number of protons. They also need to know the mass number, which is the sum of protons and neutrons. By subtracting the atomic number from the mass number, students can determine the number of neutrons. Finally, they can determine the number of electrons by assuming the atom is neutral and has the same number of electrons as protons.
Another question in the worksheet involves calculating the average atomic mass of an element based on the abundance of its isotopes. This requires students to multiply the mass of each isotope by its abundance, and then sum up these values. By dividing the sum by 100, students can calculate the average atomic mass of the element. This question helps students understand how isotopes and their abundance contribute to the overall atomic mass of an element.
The worksheet also includes questions on electron configuration, where students need to determine the arrangement of electrons in different atoms. To solve this, students must refer to the periodic table and understand the order of energy levels and sublevels. They need to fill the orbitals with electrons based on the Pauli exclusion principle and Hund’s rule. By following these steps, students can accurately determine the electron configuration of different atoms.
Overall, Unit 2 Worksheet 1 in Chemistry provides students with an opportunity to practice and deepen their understanding of various chemical concepts. By following the step-by-step solutions to the questions and problems, students can strengthen their knowledge and skills in atomic structure, isotopes, and electron configuration.