
Understanding atomic structure is essential in the study of chemistry. In Unit 3, we explore the key components of atoms and how they contribute to the overall structure. To help you check your understanding, we have provided the answer key for Unit 3 atomic structure.
The answer key includes the solutions to various questions and problems related to atomic structure. It covers topics such as the properties of protons, neutrons, and electrons, atomic number and mass number, isotopes, electron configuration, and the organization of the periodic table. By reviewing the answer key, you can ensure that you have a solid grasp of these fundamental concepts.
Using the answer key as a reference, you can compare your answers and identify any areas where you may need additional practice or clarification. It is an invaluable tool for self-assessment and can help you gauge your progress in understanding atomic structure.
Remember, mastering atomic structure is crucial for understanding the behavior of atoms and molecules, as well as the principles of chemical reactions. Therefore, make the most of this answer key and use it to enhance your knowledge and academic performance in chemistry.
Unit 3 Atomic Structure Answer Key
In Unit 3, we explored the concept of atomic structure and learned about the different components of an atom. The answer key for this unit provides the solutions to all the questions and exercises that were covered.
The answer key includes:
- A detailed explanation of the structure of an atom, including the roles of protons, neutrons, and electrons.
- Step-by-step solutions to problems related to calculating the number of protons, neutrons, and electrons in an atom.
- Examples and explanations for understanding atomic mass and atomic number.
- Explanation of isotopes and how to calculate the average atomic mass of an element.
- Answers to questions related to the organization of the periodic table and the properties of elements.
- Solutions for problems involving electron configuration and the distribution of electrons in different energy levels.
By referring to the answer key, students can check their work and ensure that they have understood the concepts correctly. It serves as a valuable tool for self-assessment and helps in improving their understanding of atomic structure.
Understanding Atomic Structure:
The concept of atomic structure is fundamental to our understanding of matter and the physical world around us. At its core, atomic structure refers to the arrangement and organization of atoms, which are the building blocks of all matter. Atoms consist of a nucleus, which contains protons and neutrons, and electrons that orbit around the nucleus in energy levels.
One of the key aspects of atomic structure is the understanding of the atomic number and mass number. The atomic number represents the number of protons in an atom, which is unique for each element. It determines the identity of an atom and is used to classify elements in the periodic table. The mass number, on the other hand, represents the sum of protons and neutrons in an atom and is used to calculate the atomic mass.
Electrons play a crucial role in atomic structure as they are responsible for the chemical properties of elements. They are arranged in energy levels or shells around the nucleus, and each energy level can hold a specific number of electrons. The distribution of electrons in these energy levels follows certain rules, such as the Aufbau principle, Hund’s rule, and the Pauli exclusion principle.
Understanding atomic structure is not only essential for studying chemistry but also for various fields of science and technology. It provides the foundation for understanding chemical reactions, bonding, and the behavior of matter at the atomic level. Moreover, advancements in atomic structure knowledge have led to important discoveries, such as the development of quantum mechanics and the creation of new materials with specific properties.
In conclusion, atomic structure is a fundamental concept in science that involves the arrangement of atoms, the role of protons, neutrons, and electrons, and the principles that govern their behavior. It is crucial for understanding the properties and behavior of matter and has significant implications in various scientific and technological fields.
Key Concepts:
In the study of atomic structure, there are several key concepts that are crucial to understanding how atoms are structured and how they behave. These concepts include:
1. Atomic Number:
The atomic number of an atom corresponds to the number of protons in its nucleus. This number determines the identity of the element and is represented by the symbol “Z”. For example, all hydrogen atoms have an atomic number of 1, indicating that they contain one proton in their nucleus.
2. Mass Number:
The mass number of an atom is the sum of the number of protons and neutrons in its nucleus. It is represented by the symbol “A”. For example, a carbon atom with 6 protons and 6 neutrons has a mass number of 12. The mass number can be used to determine the number of neutrons in an atom by subtracting the atomic number from the mass number.
3. Isotopes:
Isotopes are atoms of the same element that have different numbers of neutrons. They have the same atomic number but different mass numbers. For example, carbon-12 and carbon-14 are both isotopes of carbon. Isotopes can have slightly different properties due to their different mass numbers, such as different stabilities or radioactive decay rates.
4. Electron Configuration:
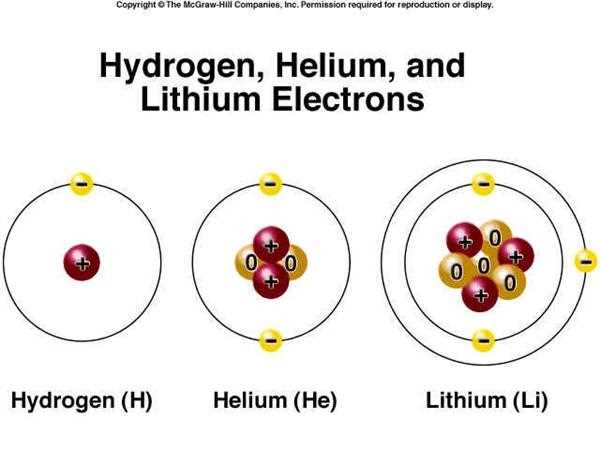
The electron configuration of an atom refers to the arrangement of electrons in its electron shells or energy levels. Electrons occupy specific orbitals within these shells, and each shell can hold a certain number of electrons. The electron configuration determines the chemical properties and behavior of an atom.
5. Valence Electrons:

Valence electrons are the electrons in the outermost energy level of an atom. These electrons are involved in chemical bonding and are responsible for the reactivity of an atom. The number of valence electrons determines the element’s position in the periodic table and its ability to form chemical bonds.
Overall, understanding these key concepts of atomic structure is essential for comprehending the behavior and properties of atoms, as well as their role in chemical reactions and bonding.
The History of Atomic Theory:
Atomic theory is the scientific theory that describes the nature and behavior of atoms. It is based on the idea that matter is made up of tiny indivisible particles called atoms. The study of atomic theory has a long and fascinating history, dating back to ancient Greek philosophy.
The ancient Greeks were the first to propose the idea of atoms. The philosopher Democritus, who lived in the 5th century BCE, believed that everything in the universe was made up of small, indestructible particles called atoms. However, his ideas were purely theoretical and lacked experimental evidence.
It wasn’t until the 19th century that the atomic theory gained widespread acceptance. In 1808, John Dalton published his atomic theory, which stated that atoms are indivisible and indestructible, and that different elements are made up of different types of atoms. Dalton’s theory laid the foundation for modern chemistry and was supported by experimental evidence.
Over time, the atomic theory continued to evolve as scientists made new discoveries. In the early 20th century, experiments by Ernest Rutherford and his colleagues led to the development of the nuclear model of the atom. Rutherford discovered that atoms have a small, dense nucleus surrounded by a cloud of electrons. This model explained the behavior of atoms and provided a more detailed understanding of atomic structure.
Today, atomic theory is a fundamental part of chemistry and physics. It has been refined and expanded upon by countless scientists and continues to shape our understanding of the natural world. The study of atomic theory has led to many important applications, including the development of new materials, technologies, and medical treatments.
In summary, the history of atomic theory is a testament to the curiosity and ingenuity of scientists throughout the ages. From the ancient Greeks to modern researchers, the quest to understand the nature of matter has led to remarkable discoveries and advancements.
The Structure of the Atom:
The structure of the atom is composed of three main subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge and are located in the nucleus, while neutrons are neutral and also found in the nucleus. Electrons, on the other hand, have a negative charge and exist in the electron cloud surrounding the nucleus. This model of the atom, known as the planetary or Bohr model, was proposed by Niels Bohr in 1913.
The nucleus, which contains the protons and neutrons, is the central part of the atom and accounts for most of its mass. The electrons, however, have negligible mass in comparison. The number of protons in an atom determines its atomic number, which defines the element. For example, an atom with 6 protons is carbon, while an atom with 29 protons is copper.
The atomic number and mass number:
- The atomic number (Z) represents the number of protons in an atom.
- The mass number (A) represents the total number of protons and neutrons in an atom.
- The number of neutrons in an atom can be calculated by subtracting the atomic number from the mass number (A-Z).
Isotopes:
Isotopes are atoms of the same element that have different numbers of neutrons. They have the same atomic number but different mass numbers. For example, carbon-12 and carbon-14 are both isotopes of carbon, with carbon-12 having 6 neutrons and carbon-14 having 8 neutrons.
Understanding the structure of the atom is crucial in various scientific fields, as it helps explain phenomena such as chemical reactions, radiation, and nuclear processes. By studying the properties and behavior of atoms, scientists can gain valuable insights into the fundamental building blocks of matter.
Atomic Models:
The concept of atomic structure has evolved over time as scientists have made new discoveries and developed new theories to explain the behavior of atoms. Early models of the atom, such as Dalton’s atomic theory, proposed that atoms were indivisible and indestructible building blocks of matter. However, as scientific knowledge advanced, it became clear that atoms were made up of smaller subatomic particles.
One of the first breakthroughs in understanding atomic structure came with the discovery of the electron by J.J. Thomson in 1897. Thomson’s “plum pudding” model proposed that electrons were embedded within a positively charged sphere, much like plums in a pudding. This model provided the first evidence of subatomic particles and challenged the idea of atoms as indivisible entities.
Later, Ernest Rutherford’s gold foil experiment in 1911 led to the discovery of the atomic nucleus. Rutherford found that alpha particles scattered at large angles, suggesting that atoms had a dense, positively charged central region. This led to the development of the planetary model of the atom, where electrons orbit a small, dense nucleus. However, this model had some limitations and could not explain certain experimental observations.
The modern understanding of atomic structure is based on quantum theory. The quantum mechanical model, also known as the electron cloud model, describes electrons as existing in regions of probability called orbitals. These orbitals have different shapes and energies, and they determine the behavior and chemical properties of an atom. The quantum mechanical model has provided a more accurate and comprehensive description of atomic structure, incorporating the wave-particle duality of electrons.
In summary, atomic models have evolved over time from the concept of indivisible atoms to the understanding of subatomic particles and the probabilistic nature of electrons. These models have been refined through experimental observations and the development of more advanced theoretical frameworks, leading to our current understanding of atomic structure.
Atomic Number and Mass Number:
The atomic number and mass number are two important properties of an atomic structure that provide key information about an atom. The atomic number represents the number of protons in the nucleus of an atom and is denoted by the symbol “Z”. It is often used to identify and classify different elements on the periodic table. For example, hydrogen has an atomic number of 1, carbon has an atomic number of 6, and oxygen has an atomic number of 8.
The mass number, on the other hand, represents the total number of protons and neutrons in the nucleus of an atom and is denoted by the symbol “A”. It is used to calculate the atomic mass of an element. The atomic mass is the weighted average of all the naturally occurring isotopes of an element. For example, carbon has a mass number of 12 because it has 6 protons and 6 neutrons in its nucleus, while carbon-14 has a mass number of 14 because it has 6 protons and 8 neutrons.
To determine the number of neutrons in an atom, we can subtract the atomic number from the mass number. This gives us the neutron number, which represents the number of neutrons in the nucleus. Neutrons are important because they contribute to the stability and behavior of an atom. Different isotopes of an element can have different numbers of neutrons, resulting in variations in their atomic mass.
In summary, the atomic number and mass number provide essential information about an atom’s identity, properties, and stability. The atomic number represents the number of protons, while the mass number represents the combined number of protons and neutrons. By understanding these concepts, scientists can better understand and manipulate the building blocks of matter.
Isotopes and Atomic Mass:
The concept of isotopes is fundamental to understanding atomic structure and the periodic table. Isotopes are different forms of the same element that have the same number of protons but different numbers of neutrons. This means that isotopes of an element have different atomic masses.
The atomic mass of an element is the weighted average of the masses of its isotopes, taking into account the relative abundance of each isotope. It is expressed in atomic mass units (amu) and can be found on the periodic table for each element. The atomic mass is an important property of an element as it is used to calculate molar mass, determine the number of atoms in a given sample, and to balance chemical equations.
To illustrate the concept of isotopes and atomic mass, let’s take the example of carbon. Carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. Carbon-12 is the most abundant isotope, accounting for about 98.9% of all carbon atoms. It has an atomic mass of 12 amu. Carbon-13 is less abundant, making up around 1.1% of carbon atoms, and has an atomic mass of 13 amu. Carbon-14 is a rare isotope found in trace amounts and has an atomic mass of 14 amu.
By taking into account the relative abundance of each isotope, the weighted average atomic mass of carbon is calculated to be around 12.011 amu. This means that on average, the carbon atoms in a sample will have a mass close to 12.011 atomic mass units. The concept of isotopes and atomic mass is crucial in understanding the properties and behavior of elements, and it plays a vital role in various fields such as chemistry, biology, and physics.