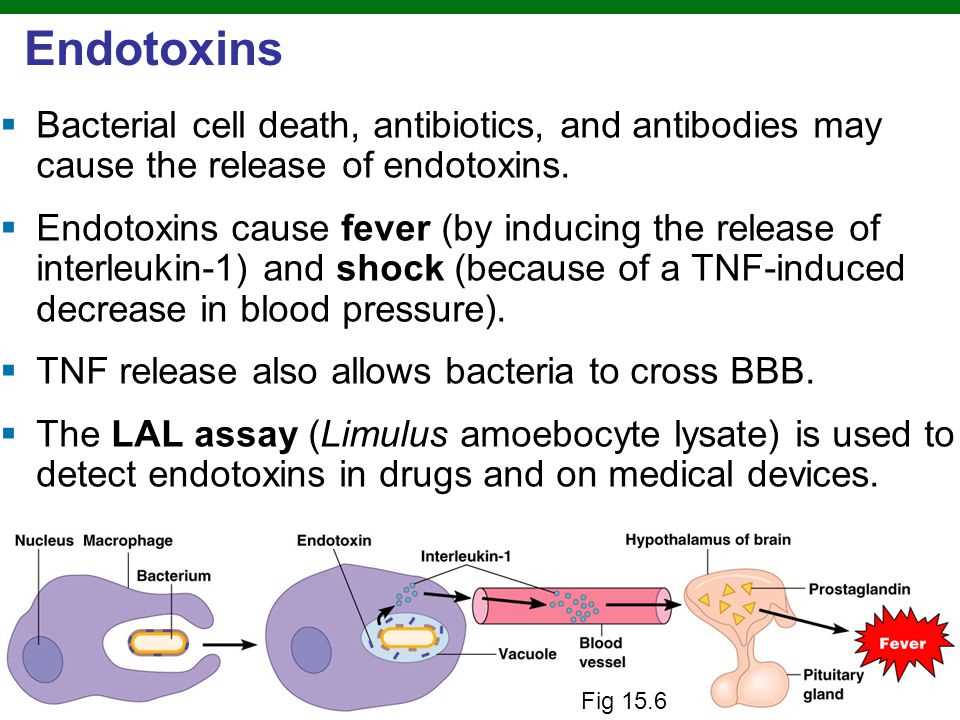
The USP 85 Bacterial Endotoxins Test is a critical procedure used in the pharmaceutical industry to determine the presence of bacterial endotoxins in drugs and medical devices. Bacterial endotoxins are toxic substances produced by certain types of bacteria, and their presence in pharmaceutical products can pose serious health risks to patients.
This test, also referred to as the Limulus amoebocyte lysate (LAL) test, is based on the properties of the horseshoe crab blood. Horseshoe crab blood contains a compound called Limulus amoebocyte lysate, which has the unique ability to detect and react to bacterial endotoxins. During the test, the LAL reagent is mixed with the pharmaceutical sample, and if bacterial endotoxins are present, a clot forms due to a cascade of reactions.
The USP 85 Bacterial Endotoxins Test is an essential part of quality control in the production of pharmaceutical products. It helps ensure that drugs and medical devices meet the required safety standards before they are released to the market. By detecting the presence of bacterial endotoxins, this test helps prevent potential harm to patients and ensures the integrity of the pharmaceutical industry.
What is the USP 85 Bacterial Endotoxins Test?
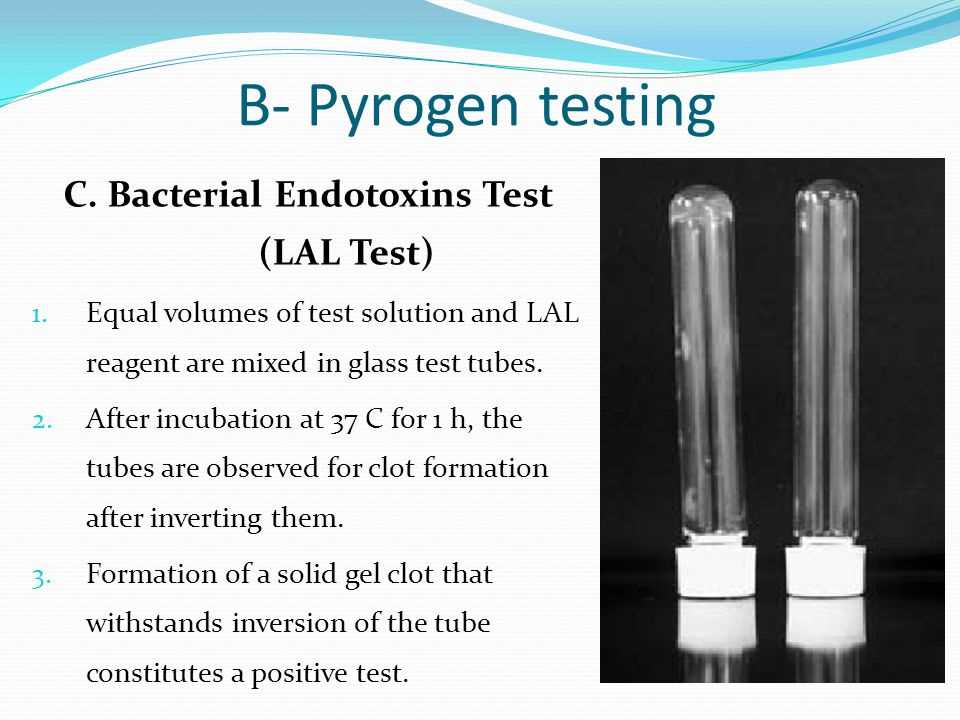
The USP 85 Bacterial Endotoxins Test, also known as the Limulus Amebocyte Lysate (LAL) test, is a method used to detect and quantify bacterial endotoxins in pharmaceutical products and medical devices. Bacterial endotoxins are lipopolysaccharides found in the outer membrane of Gram-negative bacteria. These endotoxins can cause adverse reactions in humans when present in pharmaceutical products or medical devices.
The USP 85 Bacterial Endotoxins Test is an essential test in the quality control of pharmaceutical products, as endotoxins can lead to pyrogenic reactions in patients if not removed or reduced to safe levels. Pyrogens are fever-inducing substances, and their presence in drugs or medical devices can be detrimental to patient safety.
- The test utilizes the clotting reaction of Limulus amebocyte lysate, which is derived from the blood of horseshoe crabs. This lysate reacts with bacterial endotoxins, causing gelation or clot formation.
- The USP 85 Bacterial Endotoxins Test follows strict protocols and guidelines set by the United States Pharmacopeia (USP) to ensure accurate and reliable results.
- The test can be performed using different methods, including the gel clot method, turbidimetric method, and chromogenic method. Each method has its advantages and limitations, but all aim to detect and quantify endotoxin levels.
- Proper validation and qualification of the test system, reagents, and equipment are crucial to obtaining accurate and reproducible results.
Overall, the USP 85 Bacterial Endotoxins Test plays a crucial role in ensuring the safety and quality of pharmaceutical products and medical devices. By effectively detecting and quantifying bacterial endotoxins, this test helps prevent potential harm to patients and contributes to the overall success of the healthcare industry.
Understanding the importance of endotoxin testing
Endotoxin testing plays a vital role in ensuring the safety and quality of pharmaceutical products. Endotoxins are toxic substances found in the cell walls of certain bacteria, particularly gram-negative bacteria, and they can cause severe reactions in humans, including fever, shock, and even death. Therefore, it is crucial to detect and quantify endotoxins in pharmaceutical products to prevent these adverse effects.
Implementing the United States Pharmacopeia (USP) chapter 85, also known as the Bacterial Endotoxins Test (BET), is a widely accepted method for endotoxin testing. This test involves the use of Limulus Amebocyte Lysate (LAL), a substance derived from the blood cells of the horseshoe crab, to detect and quantify endotoxins. The LAL assay takes advantage of the fact that horseshoe crab blood cells coagulate in the presence of endotoxins, providing a visually detectable reaction.
Accurate endotoxin testing is essential to ensure the safety of pharmaceutical products, particularly those administered intravenously or directly into the bloodstream. Even low levels of endotoxins can have harmful effects on patients, making it crucial to establish strict limits for endotoxin levels in pharmaceutical products. Endotoxin testing helps to validate the effectiveness of the manufacturing processes and ensure that the final product is free from endotoxins.
Understanding the importance of endotoxin testing helps pharmaceutical manufacturers meet regulatory requirements and maintain high-quality standards. It allows them to identify potential sources of endotoxin contamination in their manufacturing processes, such as contaminated raw materials or inadequate cleaning procedures. By implementing appropriate endotoxin testing procedures, manufacturers can prevent contamination and ensure the safety and efficacy of their products.
Overview of the USP 85 Bacterial Endotoxins Test
The USP 85 Bacterial Endotoxins Test is a critical method used in pharmaceutical manufacturing to determine the presence of bacterial endotoxins in raw materials, drug products, and medical devices. Bacterial endotoxins are toxic substances released by certain types of bacteria, primarily gram-negative bacteria, that can cause serious adverse reactions in humans.
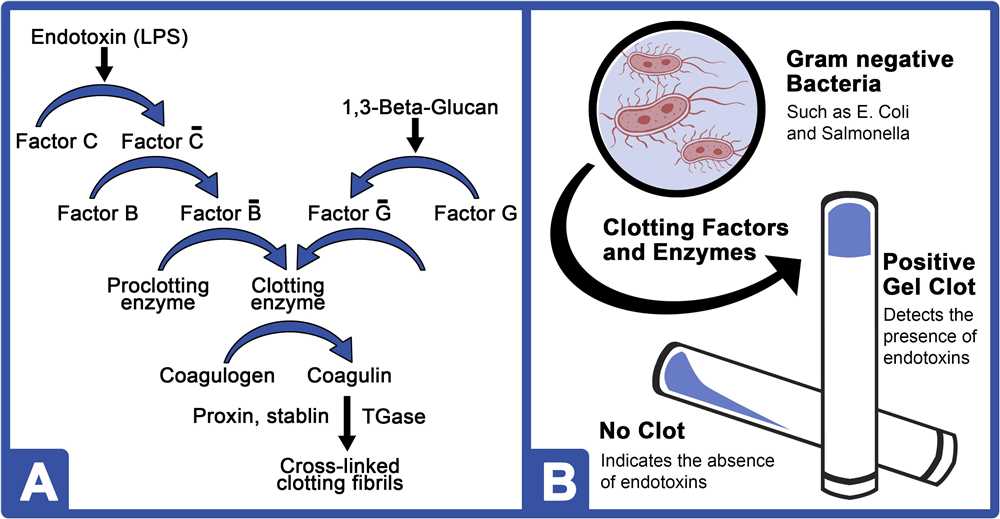
The USP 85 test is based on the detection of endotoxins using the principle of the Limulus Amebocyte Lysate (LAL) assay. The LAL reagent, derived from horseshoe crab blood, contains a proenzyme called proclotting enzyme. When endotoxins are present, they activate the proclotting enzyme, leading to a series of enzymatic reactions that result in the clotting of the LAL reagent. The clotting time is inversely proportional to the concentration of endotoxins in the test sample.
To perform the USP 85 test, the sample is first extracted using an appropriate extraction method, such as the gel-clot method or the chromogenic method. The extraction method chosen depends on the nature of the sample and the sensitivity required. After extraction, the LAL reagent is added to the sample and the mixture is incubated at a specific temperature for a set period of time. The clotting time is then measured using a spectrophotometer or visually assessed.
The USP 85 test is highly sensitive and specific, capable of detecting endotoxin levels as low as 0.001 EU/mL. It is an important quality control test in the pharmaceutical industry as endotoxins can cause pyrogenic reactions, including fever, chills, and even septic shock in patients. In addition, the presence of endotoxins can also interfere with the efficacy and stability of drug products. Therefore, ensuring the absence or acceptable levels of endotoxins is crucial for patient safety and product quality.
In conclusion, the USP 85 Bacterial Endotoxins Test provides a reliable and efficient method to detect the presence of bacterial endotoxins in pharmaceutical products. By following the USP guidelines and using validated testing methods, manufacturers can ensure the safety and quality of their products, protecting patients from potential harm caused by endotoxin contamination.
Types of endotoxin sources
The term “endotoxin” refers to a type of toxin that is produced by certain bacteria and is an integral part of their cell wall structure. These endotoxins are also known as lipopolysaccharides (LPS) and can cause a variety of harmful effects in the host organism. Endotoxins are commonly found in Gram-negative bacteria, but can also be present in certain Gram-positive bacteria and other microorganisms.
There are several different sources of endotoxins, which can vary in their composition and potency. Here are some of the main types of endotoxin sources:
- Bacterial cell walls: The primary source of endotoxins is the cell wall of Gram-negative bacteria. The outer membrane of these bacteria contains lipopolysaccharides, which are released when the bacteria are killed or undergo cell lysis. These endotoxins can then cause inflammation, immune responses, and other adverse effects in the host.
- Bacterial growth: Endotoxins can also be produced during the growth and metabolic activities of bacteria. As bacteria multiply and produce various metabolic byproducts, endotoxins can be released into the surrounding environment.
- Bacterial contamination: Endotoxins can contaminate various environments and products, such as pharmaceutical drugs, medical devices, and food. Bacterial contamination can occur during the manufacturing or handling processes, and if not properly controlled, can lead to the presence of endotoxins in the final product.
- Environmental sources: Endotoxins can also be present in the natural environment, such as water, soil, and air. This can occur due to the presence of endotoxin-producing bacteria in these environments or through contamination from other sources.
Understanding the different sources of endotoxins is important for assessing the risk of exposure and implementing appropriate testing and control measures to ensure the safety of pharmaceutical products and other materials.
Identifying key sources of endotoxins

Endotoxins, also known as lipopolysaccharides (LPS), are toxic substances produced by certain types of bacteria. They are an important concern in various industries, including pharmaceuticals, medical devices, and biotechnology, as they can cause severe adverse reactions when present in products intended for human use. Therefore, it is crucial to identify and control the key sources of endotoxins to ensure the safety and quality of these products.
One of the primary sources of endotoxins is the outer membrane of gram-negative bacteria. Gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Salmonella enterica, are common contaminants in manufacturing environments. These bacteria can contaminate the raw materials used in the production process, as well as the equipment and facilities. Therefore, regular monitoring of these potential sources is essential to prevent endotoxin contamination.
- Raw materials: Raw materials, such as water, active pharmaceutical ingredients (APIs), excipients, and packaging components, can be a significant source of endotoxins. Water used in production should undergo appropriate purification processes to remove endotoxins. APIs and excipients should be tested for endotoxin levels before use. Additionally, packaging components should be evaluated for their potential to leach endotoxins into the product.
- Equipment and facilities: Contamination can also arise from equipment and facilities used in the manufacturing process. Equipment surfaces, such as stainless steel tanks, pipelines, and filters, should be regularly cleaned and disinfected to prevent bacterial growth. The design of equipment and facilities should also consider ease of cleaning and avoidance of dead spaces where bacteria can accumulate.
- Personnel: Although less common, personnel can also be a source of endotoxin contamination. Strict hygiene practices, such as handwashing, proper gowning, and regular training on contamination control, should be implemented to minimize the risk of introducing endotoxins into the production environment.
Regular testing and monitoring of these key sources of endotoxins are critical to ensure product safety. The USP 85 Bacterial Endotoxins Test provides a method for quantifying endotoxin levels in pharmaceutical and biotechnology products. By identifying and controlling these sources, manufacturers can mitigate the risk of endotoxin contamination and ensure the quality of their products.
The Impact of Endotoxins on Pharmaceutical Products
Endotoxins are an integral part of the cell wall of Gram-negative bacteria, and they can pose significant challenges to the safety and effectiveness of pharmaceutical products. These bacterial toxins are potent and can cause severe adverse reactions in humans, including fever, septic shock, and even death. As a result, it is crucial for the pharmaceutical industry to ensure that endotoxin levels in their products are within acceptable limits, as mandated by regulatory bodies like the Food and Drug Administration (FDA).
The presence of endotoxins in pharmaceutical products can occur due to various reasons, such as contamination during manufacturing processes or inadequate sterilization techniques. Even low levels of endotoxins have the potential to elicit an immune response in patients, making it essential for pharmaceutical companies to implement effective testing methods to detect and quantify endotoxins.
One widely used method for endotoxin testing is the USP Chapter 85 Bacterial Endotoxins Test. This test provides a quantitative measure of endotoxin levels in pharmaceutical products, ensuring compliance with regulatory requirements. It involves the use of the Limulus Amebocyte Lysate (LAL) assay, which employs the clotting cascade of the horseshoe crab blood to detect endotoxins. The USP Chapter 85 provides detailed guidelines on the testing procedure, including sample preparation, assay performance, and interpretation of results.
By exploring the impact of endotoxins on pharmaceutical products and implementing rigorous testing methods like the USP Chapter 85 test, pharmaceutical companies can mitigate the risks associated with endotoxin contamination. This not only ensures the safety of patients but also helps maintain the integrity and efficacy of pharmaceutical products.
Principles behind the USP 85 Bacterial Endotoxins Test
Bacterial endotoxins are toxic substances produced by certain types of bacteria that can cause severe reactions in humans. These endotoxins are commonly found in pharmaceutical products, medical devices, and other healthcare products. To ensure the safety and efficacy of these products, it is necessary to perform the USP 85 Bacterial Endotoxins Test.
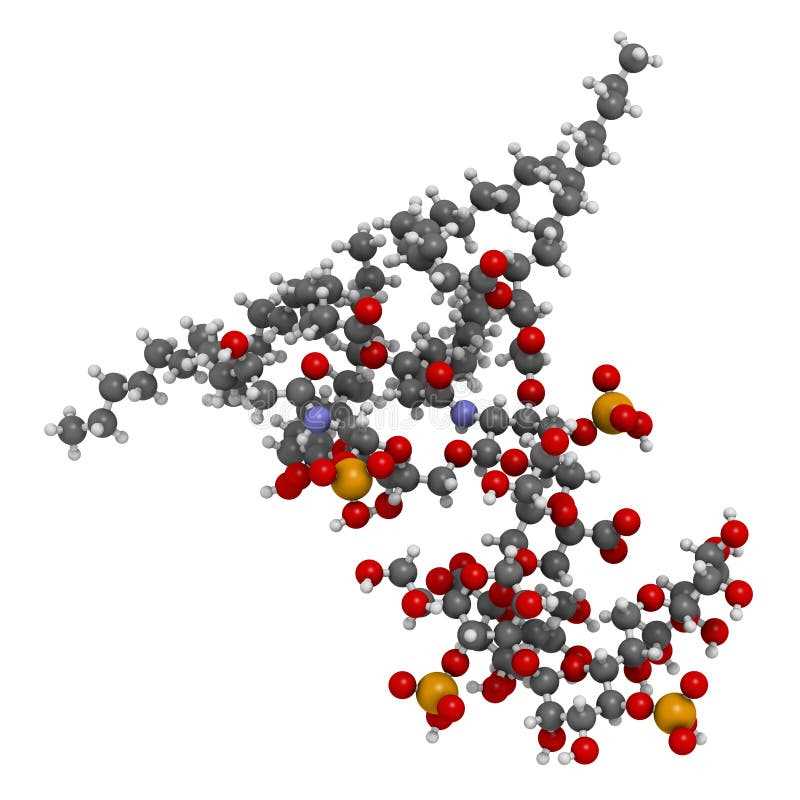
The USP 85 Bacterial Endotoxins Test is a quantitative test used to detect and measure the presence of endotoxins in various samples. This test follows a specific set of principles to ensure accurate and reliable results. One of the key principles is the use of a Limulus amebocyte lysate (LAL) reagent, derived from the horseshoe crab, which specifically reacts with endotoxins.
The test is performed by adding the LAL reagent to the sample and incubating it at a specific temperature. If endotoxins are present in the sample, they will cause a gel formation or a color change in the reaction mixture. This change is then measured using spectrophotometry or turbidimetry to quantify the amount of endotoxins present.
The USP 85 Bacterial Endotoxins Test also involves various quality control measures to ensure the reliability of the results. This includes the use of positive and negative controls, which are known samples containing specific amounts of endotoxins or no endotoxins, respectively. These controls are used to validate the test method, determine the sensitivity of the assay, and ensure consistency in the results.
Summary:
- The USP 85 Bacterial Endotoxins Test is a quantitative test used to detect and measure the presence of endotoxins in various healthcare products.
- The test relies on the use of a specific reagent derived from the horseshoe crab, known as the Limulus amebocyte lysate (LAL) reagent.
- The presence of endotoxins in the sample causes a gel formation or color change in the reaction mixture, which is measured to determine the quantity of endotoxins.
- Quality control measures, such as the use of positive and negative controls, are employed to ensure the reliability and consistency of the test results.