Water is an essential substance for life on Earth. Its unique properties make it a versatile solvent and play a crucial role in various chemical processes. The study of water and aqueous systems is important in understanding how substances dissolve, react, and interact in the presence of water.
Water molecules are polar due to the electronegativity difference between oxygen and hydrogen atoms. This polarity allows water to form hydrogen bonds with other water molecules and with other polar substances. Hydrogen bonding not only gives water its high boiling and melting points but also contributes to its cohesive and adhesive properties.
When a substance dissolves in water, it is surrounded by water molecules, which separate the individual particles of the solute and distribute them evenly throughout the solvent. This process is known as hydration. The concentration of a solute in a solution can be measured using various units, such as molarity or percent mass/volume.
Water plays a vital role in many biological processes, such as the transportation of nutrients and waste products in the bloodstream, regulation of body temperature, and support of chemical reactions in cells. Understanding the behavior of aqueous systems is essential for comprehending the chemical processes that occur in living organisms.
By studying water and aqueous systems, scientists can better understand the fundamental principles that govern chemical reactions and interactions in nature. This knowledge has applications in various fields, including medicine, environmental science, and industry. Water and aqueous systems worksheet answers provide a comprehensive overview of the concepts and principles related to water and its role in chemical processes.
Water and Aqueous Systems Worksheet Answers
In the study of chemistry, one of the fundamental concepts is understanding the behavior of water and aqueous systems. Water is a unique substance with many important properties, such as its ability to dissolve various solutes and its role as a universal solvent in biological systems. This worksheet provides answers to questions related to these concepts and helps students grasp the fundamental principles of water chemistry.
One of the main questions addressed in the worksheet is the concept of solubility. Solubility is the ability of a solute to dissolve in a solvent and form a homogeneous mixture. The worksheet provides answers to questions about factors affecting solubility, such as temperature and pressure. It also explains the concept of saturated and unsaturated solutions and how they relate to solubility.
The worksheet also covers the concept of concentration in aqueous solutions. It provides answers to questions about how to calculate the concentration of a solution using molarity and percent composition by mass. It also explains the concept of dilution and how to calculate the new concentration of a solution after dilution.
Another important topic covered in the worksheet is acids and bases. It provides answers to questions about the properties of acids and bases, such as their taste, pH scale, and reaction with indicators. The worksheet also explains the concept of neutralization reactions and provides examples of acid-base reactions and their balanced chemical equations.
In conclusion, the “Water and Aqueous Systems Worksheet Answers” provide a comprehensive understanding of the behavior of water and aqueous solutions in chemistry. It helps students grasp important concepts such as solubility, concentration, and acids and bases. By studying these answers, students can develop a strong foundation in the principles of water chemistry and apply them to various real-life scenarios.
What are Aqueous Systems?
An aqueous system is a solution in which water is the solvent. It is a homogeneous mixture where a solute, which can be a solid, liquid, or gas, is evenly distributed in water. Aqueous solutions are commonly encountered in everyday life, from the food we consume to the products we use for cleaning.
When a solute dissolves in water, it undergoes a process called hydration, in which water molecules surround the solute particles and stabilize them. This allows the solute to disperse evenly throughout the water, creating a homogeneous solution. The solute particles become solvated, meaning they are surrounded and separated by water molecules.
For example:
- When table salt, sodium chloride, dissolves in water, the sodium and chloride ions become surrounded by water molecules. The resulting solution is called a sodium chloride aqueous solution.
- When sugar, a molecular solid, dissolves in water, individual sugar molecules become surrounded by water molecules. The resulting solution is called a sugar aqueous solution.
- When carbon dioxide gas dissolves in water, carbon dioxide molecules become surrounded by water molecules. The resulting solution is called a carbon dioxide aqueous solution.
Aqueous systems play a crucial role in various chemical reactions and processes. They provide a medium for substances to react and facilitate the transportation of ions and molecules. The properties and behavior of solutes in aqueous solutions are influenced by factors such as temperature, pressure, and concentration.
Overall, aqueous systems are an essential aspect of our everyday lives and have a wide range of applications in fields such as chemistry, biology, and environmental science.
Properties of Water
Water is a unique substance that exhibits various important properties. One of the key properties of water is its high polarity. Due to its bent molecular structure and unshared electron pairs, water molecules have a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms. This polar nature of water allows it to form hydrogen bonds with other water molecules and other polar substances, contributing to its ability to dissolve many compounds.
Another important property of water is its high specific heat capacity. Water has the ability to absorb and retain large amounts of heat energy without a significant change in its own temperature. This property is essential for regulating the Earth’s climate and maintaining stable temperature conditions in living organisms. It also makes water an effective coolant in various industrial processes.
Furthermore, water exhibits a unique feature known as surface tension. Surface tension is the result of the cohesive forces between water molecules at the surface of a liquid. This property allows certain insects, like water striders, to walk on the surface of water without sinking. It also causes water droplets to form spherical shapes and enables capillary action, allowing water to rise in narrow tubes against the force of gravity.
In addition to these properties, water has a high boiling point and a relatively high density in its liquid state compared to most other substances. This is due to the hydrogen bonding between water molecules, which results in a network-like structure with closely packed molecules. These properties of water play crucial roles in the Earth’s water cycle, as well as in its ability to support life and maintain a stable environment for various organisms.
Factors Affecting Dissolution
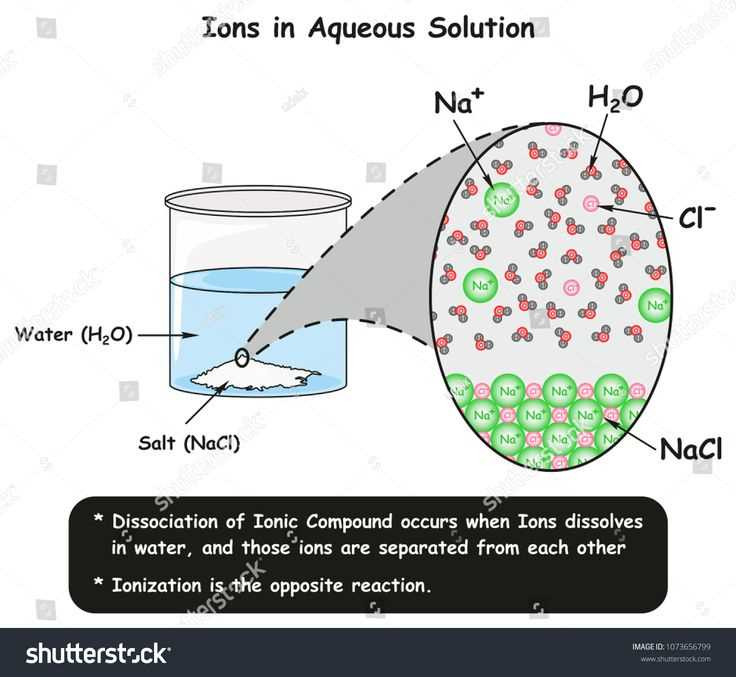
When a solute dissolves in a solvent to form a solution, there are several factors that can affect the rate and extent of dissolution. These factors include:
Nature of the Solute: The type of solute being dissolved can significantly impact its solubility in a given solvent. Some solutes are highly soluble in water, while others are not. Solubility is often determined by the polarity and molecular structure of the solute.
Nature of the Solvent: The solvent in which the solute is dissolved also plays a crucial role in the dissolution process. Some solvents are more polar than others, making them better at dissolving polar solutes. Similarly, nonpolar solvents are more effective at dissolving nonpolar solutes.
Temperature: The temperature of the solution can greatly affect the rate of dissolution. In general, increasing the temperature increases the solubility of most solutes. This is because higher temperatures provide more energy to overcome the intermolecular forces holding the solute particles together.
Pressure: The pressure applied to a solution does not have a significant effect on the solubility of most solid and liquid solutes. However, it can have an impact on the solubility of gases. According to Henry’s law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid.
Surface Area: Increasing the surface area of the solute can enhance the rate of dissolution. This is because a greater surface area allows for more contact between the solute and solvent particles, facilitating the dissolution process.
Stirring or Agitation: Stirring or agitating the solution can also accelerate the dissolution process. By continuously mixing the solute and solvent, diffusion is enhanced, leading to faster dissolution.
Overall, these factors work together to determine the extent and rate of dissolution. Understanding these factors can help in predicting and controlling the solubility of different substances in various solvents.
Concentration Units
Concentration units are used to express the amount of solute present in a given amount of solvent or solution. These units help scientists and chemists accurately measure and compare the concentration of different substances.
One commonly used concentration unit is molarity, which is expressed as moles of solute per liter of solution (mol/L). Molarity is denoted by the symbol “M” and is used to describe the number of moles of a solute dissolved in a given volume of solution. This unit is particularly useful in laboratory experiments and calculations.
Another concentration unit is percent by mass, which is expressed as the mass of the solute divided by the mass of the solution multiplied by 100. This unit is often used in the field of medicine and pharmacology to express the concentration of drugs in solutions.
In addition to these units, there are many other concentration units used in different fields of science, such as parts per million (ppm), parts per billion (ppb), and molar fraction. These units provide different ways of expressing the concentration of a solute and are used depending on the specific context and requirements of the experiment or application.
Overall, concentration units are essential in the study of water and aqueous systems as they allow scientists to quantify the amount of solute present and accurately compare different solutions. These units provide a standardized way of expressing concentration and are vital in various scientific disciplines.
Solution Stoichiometry
Solution stoichiometry is a branch of stoichiometry that deals with the quantitative relationships between reactants and products in a chemical reaction taking place in solution. It involves calculating the amounts of substances involved in a solution based on the balanced chemical equation and the known quantities of reactants or products.
When performing solution stoichiometry calculations, it is important to recognize that reactions in solution may involve ions rather than whole molecules. This means that the concentrations and stoichiometric coefficients of ions must be taken into account. The stoichiometric ratio of ions in a reaction can be determined from the balanced equation.
In solution stoichiometry, the concept of molarity is often used to express the concentration of a solute. Molarity is defined as the number of moles of solute per liter of solution. By knowing the molarity and volume of a solution, one can determine the amount of solute present.
To solve solution stoichiometry problems, one must first write a balanced chemical equation for the reaction. Then, convert the given information (such as volume or molarity) into moles using the appropriate conversion factor. Next, use the stoichiometric coefficients from the balanced equation to determine the mole ratio between the reactants and products. Finally, convert the moles of the desired substance to the desired units (such as grams or liters) if necessary.
Acid-Base Reactions in Aqueous Solutions

Acid-base reactions are a fundamental type of chemical reaction that occur in aqueous solutions. These reactions involve the transfer or exchange of hydrogen ions (H+) between substances. Acids are substances that donate hydrogen ions, while bases are substances that accept hydrogen ions.
The key characteristic of an acid-base reaction is the formation of a water molecule. When an acid donates a hydrogen ion to a base, they combine to form a water molecule. This process is known as neutralization. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) in water, they form sodium chloride (NaCl) and water (H₂O).
Acid-base reactions can also involve the exchange of other ions in addition to hydrogen ions. In some cases, the anion of the acid combines with the cation of the base to form a new compound. For example, when sulfuric acid (H₂SO₄) reacts with potassium hydroxide (KOH), they form potassium sulfate (K₂SO₄) and water.
The strength of acids and bases plays a crucial role in determining the extent to which a reaction occurs. Strong acids and bases completely dissociate in water, meaning that all of their molecules break apart into ions. This results in a more rapid and complete reaction. Weak acids and bases, on the other hand, only partially dissociate in water, resulting in a slower and less complete reaction.
Overall, acid-base reactions in aqueous solutions are important for understanding the behavior of substances in water and for balancing chemical equations. They have wide-ranging applications in various fields such as chemistry, biology, and environmental science.
Redox Reactions in Aqueous Solutions
The study of redox reactions in aqueous solutions is an important topic in chemistry. Redox reactions involve the transfer of electrons between species, resulting in the oxidation and reduction of substances. These reactions play a crucial role in various chemical and biological processes, such as cellular respiration and corrosion.
In aqueous solutions, redox reactions can occur between various species, including both ions and molecules. For example, when a metal is placed in an aqueous solution containing an oxidizing agent, such as a metal ion, the metal will undergo oxidation, losing electrons to the oxidizing agent. On the other hand, the oxidizing agent itself will be reduced, gaining electrons from the metal.
The balancing of redox reactions in aqueous solutions involves the identification of the species that undergo oxidation and reduction, as well as the determination of the number of electrons transferred. This can be accomplished using the method of half-reactions, which separates the oxidation and reduction processes into two separate equations. By balancing the number of electrons transferred in each half-reaction, the overall balanced redox equation can be obtained.
Redox reactions in aqueous solutions have numerous applications in everyday life. For example, they are used in water treatment processes to remove contaminants and disinfect water. They are also involved in the generation of electricity in batteries and the production of various chemical compounds. Understanding the principles and mechanisms of redox reactions in aqueous solutions is therefore crucial for a wide range of scientific and technological fields.