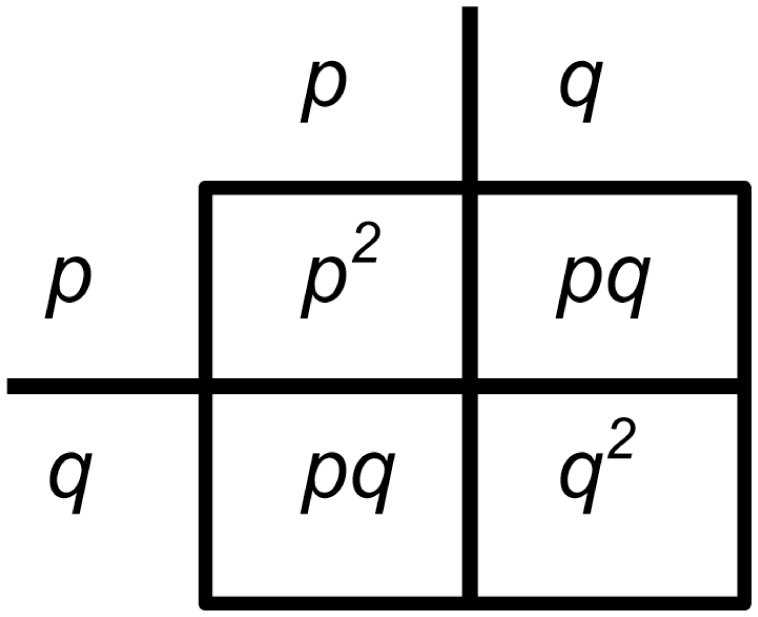
In the field of thermodynamics, work equilibrium and free energy play crucial roles in understanding the behavior of systems. Work equilibrium refers to the state in which a system is in mechanical equilibrium, meaning that the forces acting on the system are balanced. This is an important concept as it allows us to determine whether a system can do work or not.
Free energy, on the other hand, is a measure of the energy that can be extracted from a system to do useful work. It takes into account both the internal energy of the system and the entropy or disorder of the system. By understanding free energy, we can determine the spontaneity of a process and its ability to perform work.
The POGIL (Process Oriented Guided Inquiry Learning) approach to learning and teaching has become increasingly popular in recent years. POGIL activities involve students working in small groups to solve problems, discuss concepts, and draw conclusions. The purpose of this article is to provide answers and explanations to the POGIL activity on work equilibrium and free energy, helping students gain a deeper understanding of these concepts and their applications.
Work Equilibrium and Free Energy POGIL Answers
In the study of thermodynamics, it is important to understand the concepts of work equilibrium and free energy. Work equilibrium refers to the state in which the work done on a system is equal to the work done by the system. This equilibrium is achieved when the energy transfer between the system and its surroundings is balanced.
One way to determine if a system is in work equilibrium is by calculating the change in free energy. Free energy is a measure of the energy available to do work. If the change in free energy is zero, it means that the system is in work equilibrium. This can be represented mathematically by the equation ΔG = ΔH – TΔS, where ΔG is the change in free energy, ΔH is the change in enthalpy, T is the temperature, and ΔS is the change in entropy.
To solve problems involving work equilibrium and free energy, it is important to understand the concept of entropy. Entropy is a measure of the disorder or randomness of a system. If the entropy of a system increases, it means that the system is becoming more disordered. If the entropy decreases, it means that the system is becoming more ordered. When calculating the change in entropy, it is important to consider the number of particles and their distribution in the system.
Overall, understanding work equilibrium and free energy is crucial in the study of thermodynamics. By analyzing the change in free energy and considering factors such as entropy, it is possible to determine if a system is in work equilibrium and predict the direction of energy transfer.
Understanding Free Energy
Free energy is a concept used in thermodynamics to quantify the amount of work that can be extracted from a system at constant temperature and pressure. It is a measure of how useful the energy in a system is, and is often denoted by the symbol ΔG.
Understanding free energy is crucial in the study of chemical reactions and processes. The change in free energy, ΔG, can be positive or negative, indicating whether a reaction is spontaneous or non-spontaneous. A negative value for ΔG indicates that the reaction is spontaneous and can occur without the input of energy. On the other hand, a positive value for ΔG indicates that the reaction is non-spontaneous and requires the input of energy to occur.
Free energy can also be related to equilibrium, which is a state of balance in a system. At equilibrium, the forward and reverse reactions occur at the same rate, and there is no net change in the concentrations of reactants and products. The free energy change for a reaction at equilibrium, ΔG°, is often used to determine the equilibrium constant. If ΔG° is negative, the equilibrium constant is greater than 1, indicating that the reaction favors the formation of products. If ΔG° is positive, the equilibrium constant is less than 1, indicating that the reaction favors the formation of reactants.
In summary, understanding free energy is essential for comprehending the spontaneity of chemical reactions and the behavior of systems at equilibrium. It allows scientists to predict whether a reaction will occur spontaneously and provides insights into the direction of the reaction and the relative concentrations of reactants and products at equilibrium.
Importance of Work Equilibrium and Free Energy
Work equilibrium and free energy are fundamental concepts in the field of thermodynamics. Understanding these concepts is crucial for scientists and engineers across various disciplines, as they provide a framework for predicting and analyzing physical processes and chemical reactions.
Work equilibrium refers to a state where the net force acting on an object is zero, resulting in no acceleration. This state of equilibrium is important because it allows us to analyze and calculate the mechanical energy of a system. By considering the work done by and on an object, we can determine if the system is in a stable state or if any external forces are acting on it. This understanding is essential in fields such as engineering, as it allows for the design and optimization of mechanical systems.
On the other hand, free energy is a measure of the energy available to do work in a thermodynamic system. It accounts for both the internal energy of the system and the work that can be done by the system on its surroundings. Free energy is important for predicting the direction of chemical reactions and determining if a reaction is spontaneous or requires external energy input.
Overall, work equilibrium and free energy are essential concepts in the study of thermodynamics. They provide a framework for understanding and predicting physical and chemical processes, allowing scientists and engineers to make informed decisions and design efficient systems. Without a thorough understanding of work equilibrium and free energy, it would be challenging to analyze and optimize various processes in the fields of physics, chemistry, and engineering.
Key Concepts in Work Equilibrium and Free Energy
In the study of work equilibrium and free energy, there are several key concepts that are fundamental to understanding how systems behave and how energy is transferred and transformed. These concepts provide a framework for analyzing and predicting the behavior of physical and chemical processes.
Work equilibrium refers to the state in which no net work is being done on a system. This occurs when the forces acting on the system are balanced, resulting in a stable and unchanging state. The concept of work equilibrium is important in understanding how energy is conserved in various systems, and it allows us to determine when a system is in a state of equilibrium.
Free energy is a measure of the potential energy that is available to do work in a system. It represents the maximum amount of work that can be obtained from a system at a constant temperature and pressure. Free energy is useful for determining whether a reaction is spontaneous or non-spontaneous, as well as for predicting the direction in which a reaction will proceed.
Entropy is another key concept in work equilibrium and free energy. It is a measure of the degree of disorder or randomness in a system. A system with high entropy is less ordered and has more possible arrangements, while a system with low entropy is more ordered and has fewer possible arrangements. The concept of entropy is important in predicting the spontaneity of a reaction, as a spontaneous reaction typically results in an increase in entropy.
These key concepts provide a foundation for understanding the behavior of systems in equilibrium and the transfer and transformation of energy. By applying these concepts, scientists can analyze and predict the behavior of various physical and chemical processes, leading to a deeper understanding of the fundamental principles governing the natural world.
Applications of Work Equilibrium and Free Energy in Chemistry

The concepts of work equilibrium and free energy are fundamental in the field of chemistry and have a wide range of practical applications. They play a crucial role in understanding and predicting chemical reactions, as well as in designing and optimizing industrial processes.
Work equilibrium, also known as the thermodynamic equilibrium, refers to the state of a system where all competing forces and reactions are balanced. This concept is essential in determining whether a chemical reaction is feasible or not. By calculating the free energy change (∆G) of a reaction, chemists can assess whether the reaction will proceed spontaneously or if it requires external energy input to overcome the energy barrier. This information is vital in determining the optimal conditions for carrying out a reaction and maximizing the yield. For example, in the production of ammonia through the Haber-Bosch process, the knowledge of work equilibrium and free energy helps in determining the operating temperature and pressure to achieve the highest possible yield.
The principles of work equilibrium and free energy are also applied in the field of electrochemistry. The Nernst equation, which relates the potential difference of an electrochemical cell to the standard electrode potential and the concentrations of the reactants, is derived from the principles of work equilibrium and free energy. This equation is used to calculate the cell potential and predict the direction of electron flow in electrochemical reactions. It is widely used in various applications, such as batteries, fuel cells, and corrosion studies.
In industrial processes, work equilibrium and free energy are used to optimize the efficiency of chemical reactions and minimize waste. By understanding the thermodynamics of a reaction, chemists can manipulate the reaction conditions to achieve the desired outcome, such as favoring the formation of a specific product or reducing unwanted side reactions. This knowledge is particularly important in industries such as pharmaceuticals, where the production of complex organic molecules often requires precise control over reaction conditions to maximize yield and minimize cost.
In conclusion, the concepts of work equilibrium and free energy provide a foundation for understanding chemical reactions and optimizing processes in chemistry. They are essential tools in determining the feasibility of reactions, predicting the direction of electrochemical processes, and optimizing the efficiency of industrial processes. The application of these concepts has far-reaching implications, from the production of essential chemicals to the development of new medicines.
Experimental Approaches to Analyzing Work Equilibrium and Free Energy
When studying work equilibrium and free energy, experimental approaches provide valuable insights and help validate theoretical concepts. Various techniques are employed to analyze these phenomena, including calorimetry, thermodynamics, and spectroscopy.
Calorimetry is a powerful tool used to measure heat flow in a system. By accurately determining the heat transferred during a reaction, researchers can calculate the change in thermal energy and derive thermodynamic properties such as enthalpy and entropy. This information is crucial in assessing the equilibrium state and predicting whether a reaction will occur spontaneously.
Another approach involves using thermodynamics to analyze the work equilibrium and free energy of a system. Thermodynamics provides a framework to study the relationships between energy, heat, and work. By applying thermodynamic principles, scientists can calculate the free energy change of a reaction and determine if it is feasible to reach equilibrium under given conditions.
Spectroscopy is yet another experimental technique used to study work equilibrium and free energy. Spectroscopic methods such as infrared spectroscopy and nuclear magnetic resonance (NMR) spectroscopy can provide valuable information about the molecular structure and bonding involved in a reaction. By analyzing the energy levels and transitions of molecules, researchers can gain insights into the factors that affect equilibrium and free energy changes.
In summary, experimental approaches play a critical role in analyzing work equilibrium and free energy. Calorimetry, thermodynamics, and spectroscopy provide valuable tools for measuring, calculating, and understanding the energy changes and equilibrium states of a system. These techniques not only help validate theoretical concepts but also contribute to the development of practical applications in various fields such as chemistry, physics, and biochemistry.
Common Pitfalls and Challenges in Understanding Work Equilibrium and Free Energy
When studying the concepts of work equilibrium and free energy, many students encounter common pitfalls and challenges that hinder their understanding of these topics. One common pitfall is the confusion between work and equilibrium. Work refers to the transfer of energy from one object to another, while equilibrium is a state of balance where there is no net transfer of energy. It is important to distinguish between these two concepts to fully grasp the principles behind work equilibrium.
Another challenge lies in comprehending the concept of free energy. Free energy is a measure of the energy available to do work in a system. However, students often struggle to understand the relationship between free energy and equilibrium. Free energy is minimized at equilibrium, indicating that the system has reached a state of maximum stability. This concept can be difficult to grasp, as it may seem counterintuitive that maximum stability corresponds to minimal free energy.
Additionally, understanding the various factors that influence work equilibrium and free energy can be challenging. These factors include temperature, pressure, and concentration, among others. Students may find it difficult to understand how changes in these factors can affect the equilibrium state and the availability of free energy for work. A deep understanding of thermodynamics is required to fully grasp the relationships between these factors and the equilibrium state.
Furthermore, grasping the mathematical equations and calculations involved in determining work equilibrium and free energy can be another obstacle for students. These calculations involve concepts such as entropy, enthalpy, and Gibbs’ free energy equation. Students may struggle with the application of these equations and the interpretation of the results.
In conclusion, work equilibrium and free energy are complex topics that present several challenges for students. Understanding the distinctions between work and equilibrium, comprehending the concept of free energy and its relationship to equilibrium, and grasping the various factors and calculations involved are some of the common pitfalls that hinder students’ understanding. However, with dedicated study and practice, these challenges can be overcome, allowing for a deeper comprehension of these important concepts in thermodynamics.