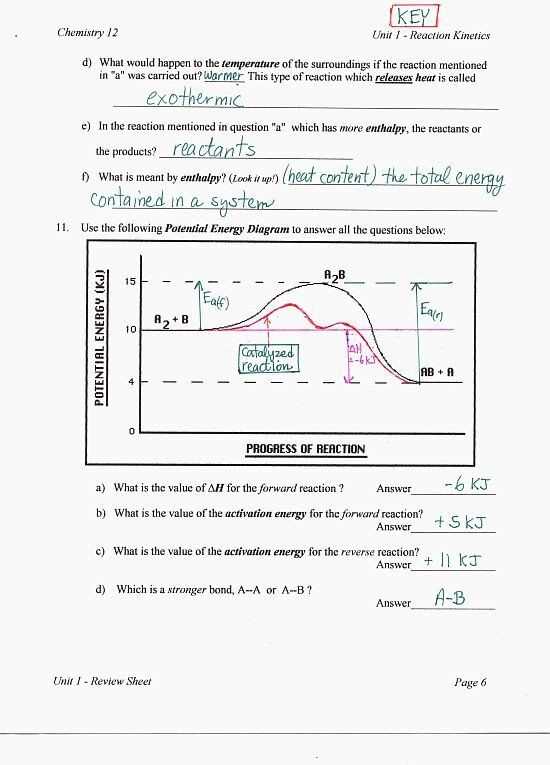
Understanding heating and cooling curves is an essential concept in thermodynamics and is crucial for understanding the behavior of substances during phase changes. In this worksheet, we will provide the answers to the questions and problems related to heating and cooling curves.
First, let’s start by discussing the heating curve. The heating curve represents the relationship between the temperature and the amount of heat absorbed by a substance as it undergoes a phase change from a solid to a liquid to a gas. It consists of different regions, each corresponding to a specific phase change. In this worksheet, we will address questions related to identifying the regions and determining the amount of heat absorbed or released at each phase change.
Next, we will move on to the cooling curve. The cooling curve represents the relationship between the temperature and the amount of heat released by a substance as it undergoes a phase change from a gas to a liquid to a solid. Similar to the heating curve, the cooling curve also consists of different regions corresponding to specific phase changes. We will answer questions related to determining the amount of heat released or absorbed during each phase change in the cooling curve.
By understanding the answers to the questions and problems in this worksheet, you will gain a better understanding of heating and cooling curves and their applications in thermodynamics. This knowledge will be valuable in various fields, such as chemistry and physics, where an understanding of phase changes is essential.
What are heating and cooling curves?
A heating curve is a graph that represents the temperature changes of a substance as heat is applied to it. It shows how the temperature of a substance increases over time as heat is added. The curve typically starts at a lower temperature and gradually increases until it reaches the substance’s boiling or melting point. During this process, the substance undergoes phase changes, such as solid to liquid or liquid to gas.
A cooling curve, on the other hand, represents the temperature changes of a substance as heat is removed from it. It shows how the temperature of a substance decreases over time as heat is transferred away. The curve typically starts at a higher temperature and gradually decreases until it reaches the substance’s freezing or condensation point. During this process, the substance undergoes phase changes in the opposite direction as in the heating curve.
Heating and cooling curves are used to study the behavior of substances during phase changes and to determine their specific heat capacities. By analyzing these curves, scientists can understand the energy transfer processes involved and the different states of matter that a substance can exist in. Additionally, heating and cooling curves are useful in various industries and applications, such as in designing heating systems, studying the properties of materials, and understanding the behavior of substances in chemical reactions.
In summary, heating and cooling curves are graphical representations of temperature changes in a substance as heat is added or removed. They provide insights into the behavior of substances during phase changes and help scientists and engineers make informed decisions in various fields.
Understanding the concept of phase change
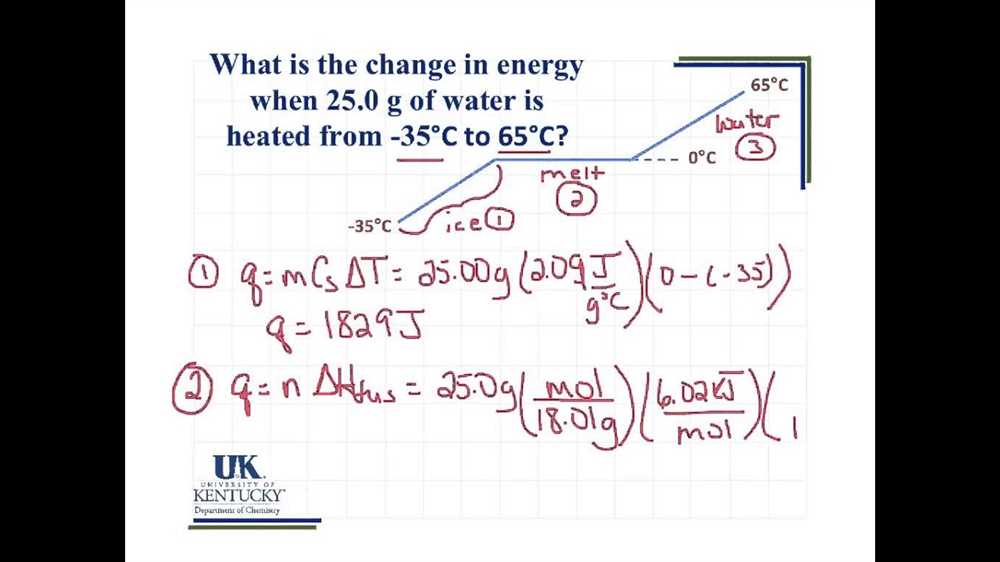
Phase change is a fundamental concept in thermodynamics that refers to the transition of matter from one state to another, such as from solid to liquid or from liquid to gas. This transition occurs when a substance reaches a specific temperature called its melting point or boiling point, depending on the direction of the phase change.
Phase change involves the exchange of energy — either heat is absorbed during the transition from a solid to a liquid or gas (known as melting or vaporization), or heat is released during the transition from a liquid or gas to a solid (known as freezing or condensation). This exchange of energy is due to the changes in the intermolecular forces between particles in different states of matter.
For example, when a solid reaches its melting point, the kinetic energy of its particles increases, causing them to vibrate more forcefully and eventually break free from their fixed positions in the crystal lattice. As the solid melts into a liquid, energy is absorbed to overcome the intermolecular forces holding the solid together. On the other hand, when a gas cools and reaches its condensation point, the particles lose kinetic energy, causing them to slow down and come closer together. As a result, the gas condenses into a liquid, releasing energy in the process.
The heating and cooling curves provide a graphical representation of the phase changes that occur as a substance is heated or cooled. These curves show the relationship between the temperature of the substance and the amount of heat absorbed or released during the phase change. By analyzing these curves, scientists and engineers can understand the behavior of substances under different temperature conditions and use this knowledge to design and optimize heating and cooling processes in various applications.
Key points:
- Phase change refers to the transition of matter from one state to another.
- Phase change involves the exchange of energy, with heat being absorbed or released during the transition.
- Heating and cooling curves provide a graphical representation of phase changes.
Factors affecting the shape of heating and cooling curves
Heating and cooling curves represent the changes in temperature over time as a substance is heated or cooled. The shape of these curves can vary depending on several factors.
1. The nature of the substance: The type of substance being heated or cooled will greatly influence the shape of the curve. Different substances have different specific heat capacities and undergo phase changes at different temperatures. For example, the heating and cooling curves of water will show distinct plateaus during the phase transitions of melting and boiling, while other substances may exhibit different behaviors.
2. Energy input or removal rate: The rate at which energy is being added or removed from the substance can also affect the shape of the curve. If heat is supplied or removed rapidly, the substance may not have enough time to reach equilibrium, resulting in a steeper curve. On the other hand, a slower energy input or removal rate allows for more time to reach equilibrium, resulting in a gentler curve.
3. Presence of impurities: Impurities present in the substance can affect its heating and cooling behavior. Impurities can raise or lower the boiling point and melting point, impacting the shape of the curves. Additionally, impurities can also affect the heat capacity of the substance, leading to variations in the curve shape.
4. Pressure conditions: The pressure under which the substance is heated or cooled can also influence the shape of the curve. Changes in pressure can result in changes in the boiling or melting points of the substance, altering the shape of the curves accordingly.
5. Experimental conditions: The experimental conditions, such as the accuracy of temperature measurements and the rate of energy transfer, can also impact the shape of the heating and cooling curves. Any variations or errors in these experimental conditions can introduce deviations in the curve shape.
In conclusion, the shape of heating and cooling curves is influenced by various factors related to the nature of the substance, the energy input or removal rate, the presence of impurities, the pressure conditions, and the experimental conditions. Understanding these factors is crucial for accurately interpreting and analyzing heating and cooling data.
Analyzing a heating curve
A heating curve is a graphical representation that shows how the temperature of a substance changes as it is heated. Analyzing a heating curve can provide valuable information about the substance’s physical properties and behavior during heating.
One important feature of a heating curve is the presence of plateaus, where the temperature remains constant even though heat is being continuously added to the substance. These plateaus correspond to phase changes, such as the melting or boiling points of the substance. By analysis of the heating curve, we can determine these phase change points and the amount of heat required for each phase change.
Temperature changes: As the substance is heated, its temperature increases steadily until it reaches a phase change point. At this point, the heat energy is being used to break the intermolecular forces holding the substance together, rather than increasing the temperature. Once the phase change is complete, the temperature starts to increase again until the next phase change point is reached. The heating curve allows us to visually see these temperature changes and identify the different phases of the substance.
Heat changes: The heating curve also provides information about the amount of heat energy required for each phase change. By measuring the length of the plateaus on the heating curve, we can calculate the heat gained or lost during each phase change using the equation Q = m * ΔH, where Q is the heat energy, m is the mass of the substance, and ΔH is the specific heat of the substance.
Overall, analyzing a heating curve provides valuable insights into the behavior of a substance during heating. It allows us to determine phase change points, understand temperature changes, and calculate the amount of heat energy involved in each phase change.
Analyzing a cooling curve
A cooling curve is a graphical representation of the temperature changes that occur during the process of cooling a substance. It is typically plotted with time on the horizontal axis and temperature on the vertical axis. By analyzing a cooling curve, we can gain insights into the behavior of the substance as it goes through the cooling process.
One key observation we can make from a cooling curve is the presence of different phases. As the substance cools, it may transition from one phase to another, such as from a gas to a liquid or from a liquid to a solid. Each phase transition is characterized by a plateau in the cooling curve, where the temperature remains constant despite the passage of time. These plateaus correspond to the energy required or released during the phase transition.
Another aspect to analyze in a cooling curve is the rate of cooling. By examining the slope of the cooling curve at different points, we can determine the rate at which the substance is losing heat. A steep slope indicates rapid cooling, while a shallow slope suggests slower cooling. This information can be useful in various applications, such as understanding the efficiency of cooling systems or predicting the behavior of materials during solidification processes.
In addition to analyzing the cooling curve itself, it is also common to compare experimental cooling curves with theoretical curves or known cooling curves for similar substances. This allows for further understanding of the substance’s behavior and can help identify any deviations or anomalies. Overall, analyzing a cooling curve provides valuable insights into the cooling process and the properties of the substance being cooled.
Interpreting the results of a heating and cooling curve experiment
In a heating and cooling curve experiment, the temperature of a substance is measured as it is heated or cooled over time. The results of this experiment can provide valuable information about the substance’s properties and behavior during phase changes. By analyzing the heating and cooling curves, scientists can determine the substance’s melting point, boiling point, heat of fusion, and heat of vaporization.
One key aspect of interpreting the results of a heating and cooling curve experiment is understanding the different phases and phase transitions of the substance. During heating, the substance goes through a solid phase, a phase transition to a liquid phase at the melting point, and then a phase transition to a gaseous phase at the boiling point. Similarly, during cooling, the substance undergoes phase transitions from a gas to a liquid and then from a liquid to a solid.
To interpret the heating curve, it is important to analyze the temperature changes at different points. The slope of the curve will be steeper during the solid-to-liquid phase transition as heat is absorbed to break the intermolecular forces holding the solid together. Once the substance reaches the liquid phase, the temperature will remain constant until all the solid has melted. This plateau is known as the melting point. After the melting point, the heating curve will show a gradual increase in temperature until it reaches the boiling point, at which another plateau will be observed. This plateau represents the phase transition from liquid to gas as heat is absorbed to break the intermolecular forces between molecules.
The cooling curve, on the other hand, shows the opposite trend. The temperature gradually decreases as the substance transitions from a gas to a liquid, and then from a liquid to a solid. The cooling curve will have plateaus at the boiling point and the melting point, similar to the heating curve. By examining these plateaus, scientists can determine the boiling point and melting point of the substance. Additionally, the slope of the cooling curve during the liquid-to-solid phase transition can be used to calculate the heat of fusion.
In conclusion, the heating and cooling curve experiment provides valuable insights into the properties and behaviors of substances during heating and cooling. By analyzing the curves, scientists can determine various properties such as melting point, boiling point, and heat of fusion. This information is crucial in understanding the thermodynamic properties of materials and can be applied in various fields, including chemistry, materials science, and engineering.
Common errors in heating and cooling curve experiments
Heating and cooling curve experiments are commonly performed to study the behavior of substances during phase changes. However, there are several common errors that can affect the accuracy of the results obtained. It is important to be aware of these errors and take necessary precautions to minimize their impact.
One common error is inaccurate temperature measurement. Temperature sensors used in the experiment may not provide precise measurements, leading to errors in recording the data. It is essential to calibrate the temperature sensors properly and ensure their accuracy before conducting the experiment. Additionally, it is crucial to take multiple readings at different points in the substance to account for any variations in temperature within the sample.
Another common error is the heat loss or gain by the surroundings during the experiment. In an ideal heating or cooling curve experiment, the heat input or output should only be affecting the sample being studied. However, in reality, there is always some heat exchange with the surroundings, which can lead to errors in the observed temperature changes. To minimize this error, it is important to insulate the experimental setup properly and reduce any heat transfer to or from the surroundings.
Furthermore, errors may arise from incorrect data analysis and interpretation. It is crucial to correctly identify the phase changes in the heating and cooling curves and analyze the data accordingly. Failure to do so can lead to inaccurate conclusions about the substance’s behavior during phase changes. Additionally, it is important to account for any experimental uncertainties and errors in the data analysis process, and calculate the overall uncertainty associated with the results.
In conclusion, conducting heating and cooling curve experiments can be a valuable tool for studying phase changes. However, it is important to be aware of common errors that can arise during these experiments and take necessary precautions to minimize their impact on the accuracy of the results obtained. Accurate temperature measurement, minimizing heat loss or gain, and careful data analysis are essential steps to ensure reliable and meaningful results.