
Chemical equations play a crucial role in understanding and predicting chemical reactions. They provide a concise and systematic way to represent the transformation of reactants into products. This worksheet is designed to help students practice writing chemical reaction formula equations and understand key concepts involved in balancing and interpreting these equations.
The worksheet includes a series of chemical reactions that students need to balance and write the corresponding formulas for. Each reaction involves different types of compounds, such as acids, bases, and salts. Students are required to identify the reactants and products, determine the correct coefficients to balance the equation, and write the final equation.
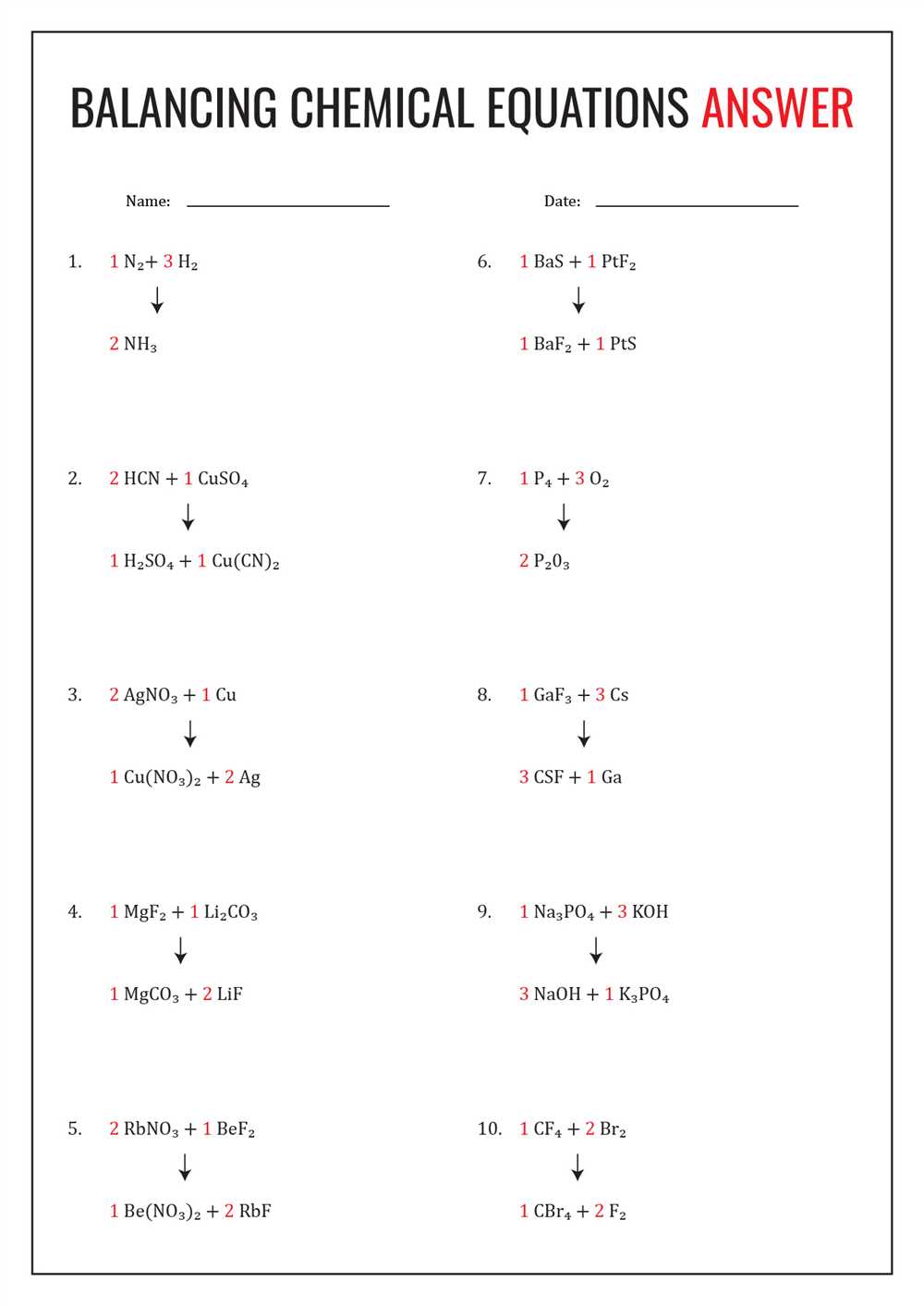
This answer key provides students with the correct responses to the worksheet exercises. It serves as a helpful resource for self-assessment and allows students to compare their own answers with the key provided. By reviewing the answer key, students can identify any mistakes or areas for improvement in their understanding of chemical reaction formula equations.
Worksheet 1 Writing Chemical Reaction Formula Equations Answer Key
Below is the answer key for Worksheet 1, which focuses on writing chemical reaction formula equations. This worksheet is a helpful tool for students to practice and reinforce their understanding of how to properly write chemical equations.
Question 1:
Write the balanced chemical equation for the reaction between potassium hydroxide (KOH) and hydrochloric acid (HCl).
Answer:
KOH + HCl → KCl + H₂O
Question 2:
Write the balanced chemical equation for the reaction between calcium carbonate (CaCO₃) and hydrochloric acid (HCl).
Answer:
CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂
Question 3:
Write the balanced chemical equation for the reaction between sodium hydroxide (NaOH) and sulfuric acid (H₂SO₄).
Answer:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
Overall, this worksheet provides students with the opportunity to practice writing chemical reaction formula equations and reinforces their understanding of balancing equations. Through this exercise, students can strengthen their skills in representing chemical reactions in a concise and accurate manner.
Understanding Chemical Reaction Formula Equations
Chemical reaction formula equations are expressions that represent the changes that occur during a chemical reaction. These equations provide a concise and standardized way to describe the reactants, products, and stoichiometry of a reaction. By understanding these equations, scientists can predict and manipulate chemical reactions, which is essential for fields such as chemistry and material science.
A typical chemical reaction formula equation consists of two parts: the reactants and the products. The reactants are the substances that undergo changes, while the products are the new substances formed as a result of the reaction. The reactants are written on the left side of the equation, and the products are written on the right side.
The equation is balanced to ensure that the law of conservation of mass is satisfied. This means that the number of atoms of each element must be the same on both sides of the equation. To balance the equation, coefficients are added in front of the reactants or products. These coefficients represent the relative amounts of each substance involved in the reaction.
For example, the combustion of methane can be represented by the equation:
CH4 + 2 O2 → CO2 + 2 H2O
In this equation, methane (CH4) reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). The equation is balanced by adding a coefficient of 2 in front of both the oxygen and water molecules.
Understanding chemical reaction formula equations allows scientists to study and analyze reactions more effectively. These equations help in determining reaction rates, understanding chemical kinetics, and predicting the outcomes of reactions. They also serve as a foundation for stoichiometry, the quantitative relationship between the amounts of reactants and products involved in a chemical reaction.
Importance of Worksheet 1
The Worksheet 1 on writing chemical reaction formula equations is an essential tool for students learning chemistry. It provides a structured format for practicing the fundamental skill of writing chemical equations. By completing this worksheet, students can strengthen their understanding of chemical reactions and improve their ability to accurately represent these reactions in equation form.
This worksheet allows students to apply their knowledge of chemical formulas and the rules of chemical reactions in a hands-on manner. It presents various scenarios and asks students to identify the reactants and products, and then write the balanced equation. This process helps students develop critical thinking skills, as they must consider the conservation of mass and the presence of any catalysts or stoichiometric coefficients.
The Worksheet 1 also serves as a valuable assessment tool for teachers. It allows them to gauge the understanding and proficiency of their students in writing chemical equations. By reviewing the completed worksheets, teachers can identify any misconceptions or areas of weakness that need further clarification in future lessons.
Additionally, the Worksheet 1 can be used as a springboard for further exploration of chemical reactions. Students can analyze the equations they have written and evaluate the types of reactions involved, such as combustion, synthesis, or decomposition. They can also delve into the concept of limiting reactants and discuss the concept of theoretical and actual yields.
In conclusion, the Worksheet 1 on writing chemical reaction formula equations plays a vital role in the learning process of chemistry students. It provides a structured format for practicing and reinforcing the essential skill of writing chemical equations accurately. It also serves as a useful assessment tool for teachers, enabling them to gauge student understanding and identify areas for improvement. Overall, this worksheet helps students build a strong foundation in chemistry and prepares them for more advanced topics in the subject.
Step-by-Step Guide to Worksheet 1
In the Worksheet 1 on writing chemical reaction formula equations, you will learn how to write balanced chemical equations based on given reaction scenarios. The key objective of this exercise is to understand the process of formulating chemical equations accurately.
Step 1: Read the given reaction scenario carefully. Identify the reactants and products involved in the chemical equation. Understand the changes that occur during the reaction.
Step 2: Determine the chemical symbols of the substances involved in the reaction. Refer to the periodic table if needed. Write down the formulas of the reactants and products using the correct symbols and subscripts.
Step 3: Balance the equation by adjusting the coefficients in front of each compound. Ensure that the number of atoms on both sides of the equation is equal. This step is crucial to maintain the law of conservation of mass.
Step 4: Check the balanced equation for accuracy. Ensure that all elements are balanced and the equation follows the rules of chemical formulas and equations.
For example, if the given scenario states that potassium hydroxide reacts with hydrochloric acid to produce potassium chloride and water, you would start by identifying the reactants (potassium hydroxide and hydrochloric acid) and products (potassium chloride and water). Then, you would write the formulas of each compound: KOH + HCl → KCl + H2O. Next, you would balance the equation: 2KOH + 2HCl → 2KCl + 2H2O. Finally, you would check if the equation is balanced and accurate.
By following these step-by-step instructions, you can successfully complete Worksheet 1 and gain a better understanding of how to write chemical reaction formula equations.
Sample Chemical Reaction Problems
Chemical reaction problems are an important aspect of chemistry, as they allow us to understand and predict the behavior of different substances. By writing and balancing chemical equations, we can determine the reactants and products involved, as well as the ratios in which they combine.
Problem 1:
Write the balanced chemical equation for the reaction between sodium and chlorine gas to form sodium chloride. Sodium has an atomic number of 11, while chlorine has an atomic number of 17.
Solution:
The balanced chemical equation for this reaction is:
- 2Na + Cl2 → 2NaCl
This equation shows that two atoms of sodium combine with one molecule of chlorine gas to form two molecules of sodium chloride.
Problem 2:
Balance the chemical equation for the reaction between methane and oxygen gas to produce carbon dioxide and water. Methane has the formula CH4.
Solution:
The balanced chemical equation for this reaction is:
- CH4 + 2O2 → CO2 + 2H2O
This equation shows that one molecule of methane reacts with two molecules of oxygen gas to produce one molecule of carbon dioxide and two molecules of water.
Problem 3:
Write and balance the chemical equation for the reaction between magnesium and hydrochloric acid to form magnesium chloride and hydrogen gas. Magnesium has an atomic number of 12.
Solution:
The balanced chemical equation for this reaction is:
- Mg + 2HCl → MgCl2 + H2
This equation shows that one atom of magnesium reacts with two molecules of hydrochloric acid to form one molecule of magnesium chloride and one molecule of hydrogen gas.
Common Mistakes to Avoid
When writing chemical reaction formula equations, it is important to avoid common mistakes in order to accurately represent the reactions occurring. By recognizing and avoiding these mistakes, one can ensure the clarity and correctness of the equation.
1. Incorrect balancing: One common mistake is failing to properly balance the equation. Balancing is crucial as it ensures the conservation of mass and charge. Take the time to carefully balance all elements and charges on both sides of the equation.
2. Inconsistent states of matter: Another mistake to avoid is representing the states of matter inconsistently in the equation. Include the appropriate state (solid, liquid, gas, or aqueous) using the correct symbols. This helps convey the conditions under which the reaction occurs.
3. Omission of important information: It is essential to include all necessary information in the equation. Avoid omitting important details such as the physical conditions (temperature and pressure) or the catalyst, if applicable. These details enable others to replicate the reaction under similar conditions.
4. Incorrect use of coefficients: Coefficients are used to indicate the number of molecules or atoms involved in a reaction. Avoid using incorrect coefficients that do not accurately represent the reaction. Take the time to balance the equation properly and use the appropriate coefficients.
- 5. Incorrect use of chemical formulas: It is essential to use the correct chemical formulas in the equation. Avoid using incorrect formulas for compounds or elements, as this can lead to inaccurate representations of the reaction. Double-check the formulas for accuracy.
- 6. Lack of clarity: Clarity is important when writing chemical equations. Avoid using ambiguous or unclear language. Use clear and concise terms to accurately describe the reactants, products, and conditions of the reaction.
- 7. Failure to account for charges: Some reactions involve ions, which carry charges. It is important to properly represent these charges in the equation. Failure to do so can result in an incorrect representation of the reaction. Include charges when necessary.
- 8. Mixing up reactants and products: Another mistake to avoid is mixing up the reactants and products in the equation. Reactants should always be written on the left side of the equation, and products on the right side. Pay attention to the order and placement of substances in the equation.
By being aware of these common mistakes and taking the time to avoid them, one can ensure the accuracy and clarity of chemical reaction formula equations.
Tips for Writing Chemical Reaction Formula Equations
Writing chemical reaction formula equations can be a challenging task, but with some guidance, it can become easier. Here are some tips to help you write accurate chemical reaction formula equations:
- Identify the reactants and products: Before writing the equation, identify the substances that are participating in the reaction and those that are produced. This will help you determine the correct formulas to use.
- Balance the equation: It is important to balance the equation to ensure that the number of atoms on both sides of the equation is equal. This can be done by adding coefficients in front of the formulas.
- Use correct chemical formulas: Make sure to use the correct chemical formulas for the reactants and products. This can be done by referring to a periodic table or other reliable sources.
- Indicate the states of matter: It is important to indicate whether the substances are in a solid (s), liquid (l), gas (g), or aqueous (aq) state. This information can be written next to the formula.
- Include appropriate reaction conditions: If the reaction requires specific conditions such as temperature, pressure, or the presence of a catalyst, it is important to include this information in the equation.
- Check for consistency: Double-check the equation to ensure that it is consistent with the law of conservation of mass. This means that the total number of atoms of each element should be the same on both sides of the equation.
By following these tips, you can write accurate and balanced chemical reaction formula equations. Practice and familiarity with different types of reactions will also help improve your skills in writing these equations.