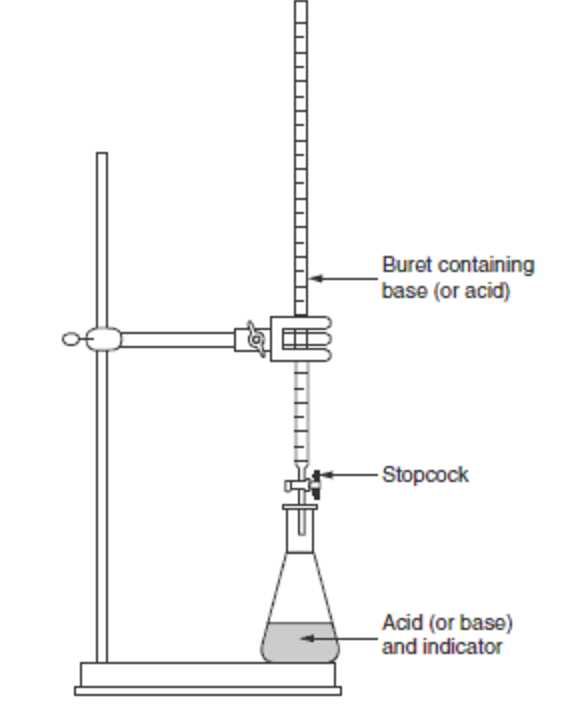
Acid-base titration is a common experiment performed in chemistry labs to determine the concentration of an unknown acid or base solution. This type of experiment involves the reaction of an acid with a base, usually using an indicator to visually determine when the reaction is complete.
The purpose of this experiment is to help students understand the concept of titration and strengthen their understanding of acid-base reactions. By carefully measuring and mixing solutions of known concentration and using the principles of stoichiometry, students can calculate the concentration of an unknown solution.
In this lab, students are usually provided with a solution of known concentration, called the titrant, and an unknown solution that needs to be analyzed. The titrant is slowly added to the unknown solution while the student observes for changes in color or pH. When the reaction is complete, the student records the volume of titrant used.
Using the volume of titrant and the known concentration, students can calculate the concentration of the unknown solution using the formula C1V1 = C2V2, where C1 is the concentration of the titrant, V1 is the volume of titrant used, C2 is the concentration of the unknown solution, and V2 is the volume of the unknown solution.
Lab Answers for Acid-Base Titration
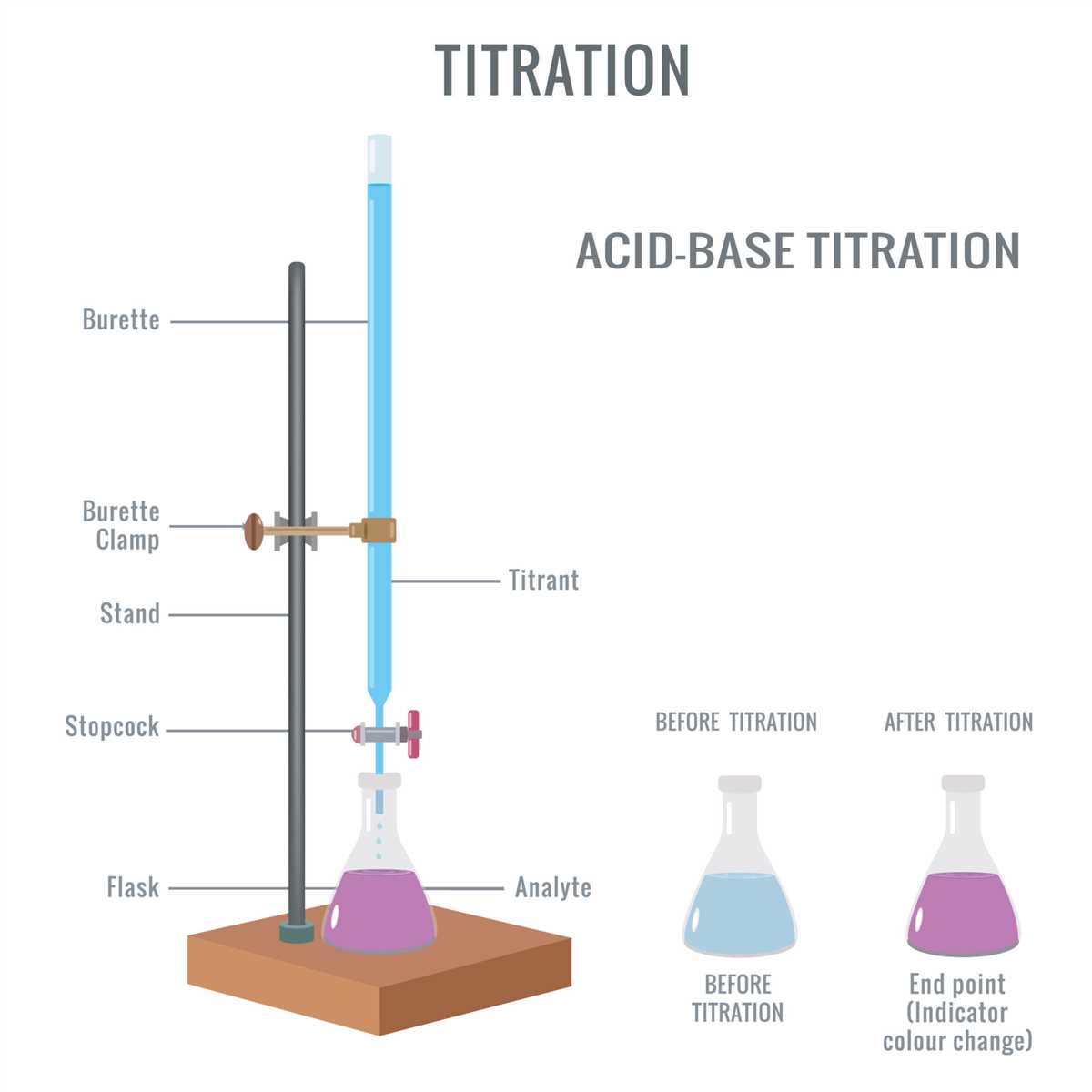
Acid-base titration is a common laboratory technique used to determine the concentration of an acidic or basic solution. In this experiment, a known concentration of acid or base, called the titrant, is slowly added to a solution of unknown concentration, called the analyte. The reaction between the titrant and analyte is monitored using an indicator, which changes color at the equivalence point, where the moles of acid and base are stoichiometrically equivalent.
One common question in an acid-base titration lab is determining the stoichiometric ratio between the acid and base. To do this, the balanced chemical equation for the reaction between the acid and base is needed. From this equation, the ratio of moles of acid to base can be determined. For example, if the balanced equation is HCl + NaOH → NaCl + H2O, the ratio is 1:1, meaning one mole of acid reacts with one mole of base.
Another question that arises in an acid-base titration lab is finding the concentration of the analyte. This can be calculated using the stoichiometric ratio determined earlier and the volume of the titrant needed to reach the equivalence point. The equation C1V1 = C2V2 can be used, where C1 is the concentration of the titrant, V1 is the volume of titrant used, C2 is the concentration of the analyte, and V2 is the volume of analyte. By rearranging the equation, the concentration of the analyte, C2, can be solved for.
Key Points:
- Acid-base titration is a technique used to determine the concentration of an acidic or basic solution.
- The stoichiometric ratio between the acid and base is determined from the balanced chemical equation.
- The concentration of the analyte can be calculated using the stoichiometric ratio and the volume of titrant used.
What Is Acid-Base Titration?
Acid-base titration is a common laboratory technique used in chemistry to determine the concentration of an acid or a base solution. It involves a controlled reaction between an acid and a base of known concentration, with the goal of finding the exact point at which the reaction is complete. This point, known as the equivalence point, is reached when the number of moles of acid is equal to the number of moles of base present in the solution.
In an acid-base titration, a solution of a known concentration, called a standard solution, is slowly added to a solution of an unknown concentration using a burette. The solution of unknown concentration is referred to as the analyte. A few drops of an indicator, such as phenolphthalein or methyl orange, are added to the analyte to visually indicate when the reaction is complete.
During the titration process, small amounts of the standard solution are added to the analyte, which causes a chemical reaction to occur. This reaction usually involves the transfer of protons (H+) from the acid to the base. The reaction continues until the equivalence point is reached, which is when the indicator changes color. At this point, the moles of acid and base are equal, allowing for the calculation of the concentration of the unknown solution.
Titrations can be used to determine the concentration of various acids and bases, as well as to identify unknown substances in a solution. Acid-base titration is a precise and accurate method that relies on the principles of stoichiometry and chemical reactions to yield quantitative results. It is commonly used in analytical chemistry, environmental science, and pharmaceutical research to study and analyze the properties of different substances in solution.
The Purpose of Acid-Base Titration Lab
Acid-base titration is a common laboratory technique used to determine the concentration of an unknown acidic or basic solution by reacting it with a standardized solution of known concentration. The purpose of performing acid-base titration is to accurately determine the concentration of a specific solute in a solution and to understand the concept of stoichiometry and molar ratios between reactants and products.
The acid-base titration lab involves carefully measuring and dispensing the unknown solution into a flask, adding an indicator that changes color at the equivalence point, and then slowly adding the standardized solution until the color change occurs. The point at which the color changes indicates that the reaction between the acid and base is complete and stoichiometrically balanced. This point, known as the equivalence point, helps determine the concentration of the unknown solution.
The purpose of the acid-base titration lab is threefold:
- To determine the concentration of an unknown acidic or basic solution: By carefully measuring the volume of the standardized solution needed to reach the equivalence point, it is possible to calculate the concentration of the unknown solution using the concept of stoichiometry.
- To practice and understand stoichiometry: This laboratory technique helps students understand the concept of stoichiometry and the molar ratios between reactants and products. By carefully measuring the volumes of solutions and understanding the balanced chemical equation, students can apply stoichiometric calculations to determine the concentration of the unknown solution.
- To improve laboratory technique and accuracy: Acid-base titration requires precise and careful measurements of solutions. Through this lab, students can develop their laboratory skills, such as pipetting, measuring volumes, and handling solutions accurately. It also teaches them to make precise observations, identify color changes, and recognize the equivalence point, which enhances their overall experimental accuracy.
Procedure for Acid-Base Titration Lab
In the Acid-Base Titration Lab, the goal is to determine the concentration of an unknown acid or base solution by reacting it with a solution of known concentration. The procedure for this lab involves several steps to ensure accurate results.
Step 1: Preparation
First, gather all the necessary equipment and chemicals for the experiment. This includes a burette, pipette, standardized solution of known concentration, indicator, and the unknown solution. Make sure all equipment is clean and calibrated before use.
Step 2: Standardization of the Solution
To start the experiment, the solution of known concentration needs to be standardized. This is done by titrating a known volume of the solution with a solution of the standardized concentration, using an indicator to determine the endpoint. Repeat this process at least three times to ensure accuracy.
Step 3: Titration of the Unknown Solution
After standardizing the solution, it is time to titrate the unknown solution. Fill the burette with the standardized solution and use a pipette to measure a known volume of the unknown solution into a flask. Add a few drops of indicator to the flask.
Step 4: Titration Process
Slowly add the standardized solution from the burette to the flask, swirling the flask gently to mix the solution. Watch for a color change in the flask, indicating the endpoint of the titration. This color change occurs when the acid-base reaction is complete.
Step 5: Data Collection
Record the volume of the standardized solution used to reach the endpoint. Repeat the titration process at least three times to ensure precision. Calculate the average volume used and use it in the calculation to determine the concentration of the unknown solution.
Step 6: Cleanup
After completing the titration process, clean all equipment thoroughly to prevent contamination. Dispose of any leftover solutions properly according to the lab’s guidelines.
In conclusion, the procedure for the Acid-Base Titration Lab involves preparing the equipment and chemicals, standardizing the solution, titrating the unknown solution, collecting data, and cleaning up. Following these steps carefully ensures accurate and reliable results in determining the concentration of the unknown solution.
Data Collection and Analysis
During the acid-base titration lab, we collected data to determine the concentration of an unknown acid solution. The titration involved adding a base solution to the acid solution until the reaction reached its equivalence point, indicated by a color change. We recorded the initial and final volumes of the base solution added and calculated the volume of base solution needed to neutralize the acid solution.
The data collected from the titration allowed us to analyze the concentration of the unknown acid solution. By using stoichiometry, we could determine the moles of acid present in the solution. We then used the volume of the acid solution and the moles of acid to calculate the concentration in units of moles per liter (M).
To ensure accurate data collection, we followed proper lab techniques and used precise measurement tools, such as burettes and pipettes. It was important to carefully read and record the initial and final volumes of the base solution to accurately calculate the volume of base needed for neutralization. Any spills or mistakes in data collection could have led to inaccurate results.
Data Analysis
After collecting the data, we plotted a graph of the volume of base solution added versus the pH of the solution. This graph allowed us to determine the equivalence point, where the pH rapidly changed. The equivalence point indicated that the acid and base had reacted completely and neutralized each other.
Using the volume of base solution at the equivalence point, we could calculate the concentration of the acid solution. We used the equation C1V1 = C2V2, where C1 is the concentration of the base solution, V1 is the volume of the base solution at the equivalence point, C2 is the concentration of the acid solution, and V2 is the volume of the acid solution.
By analyzing the collected data and performing calculations, we were able to determine the concentration of the unknown acid solution. This information is important for understanding the properties and behavior of the acid in various chemical reactions.
Determination of Unknown Concentration
During an acid-base titration lab, the primary goal is to determine the unknown concentration of an acid or base solution. This is done by slowly adding a standardized solution of either acid or base (known as the titrant) to the solution with unknown concentration (known as the analyte) until the equivalence point is reached.
Before beginning the titration, it is necessary to prepare the analyte solution of known volume. This can be done by carefully measuring a specific volume of the analyte using a graduated cylinder or pipette. It is crucial to accurately measure the volume, as any deviation can lead to incorrect results. Additionally, it is essential to record the initial volume of the analyte solution to calculate the volume of titrant required to reach the equivalence point.
During the titration, the titrant is added slowly while continuously monitoring the change in pH of the analyte solution. This can be done using a pH probe or indicator. As the titrant is added, the pH of the solution gradually changes until it reaches a sudden and significant change known as the equivalence point. At this point, the moles of acid or base in the analyte solution are equal to the moles of acid or base in the titrant solution, allowing for the calculation of the unknown concentration.
- To determine the unknown concentration, the volume of titrant required to reach the equivalence point is recorded.
- Using the known volume and concentration of the titrant, the moles of titrant can be calculated.
- Using the balanced chemical equation for the reaction between the acid and base, the moles of acid or base in the analyte solution can be determined.
- Finally, the unknown concentration of the acid or base in the analyte solution can be calculated by dividing the moles of acid or base by the volume of the analyte solution.
It is important to note that the accuracy of the determination of unknown concentration depends on several factors, including the precision of volume measurements, the accuracy of the titrant concentration, and the stability of the titration conditions. Careful attention to these factors can help ensure reliable and accurate results.
Sources of Error and Improvements
During the acid-base titration lab, several sources of error may have impacted the accuracy of the results. It is important to identify and address these sources of error in order to improve the reliability of the experiment.
Human Error: Human error can occur during the measurement of volumes, the addition of reagents, and the recording of data. To minimize human error, it is important to carefully read the markings on the volumetric glassware, take measurements at eye level, and practice proper technique when adding reagents. Additionally, having multiple individuals perform the experiment and comparing results can help identify and correct any potential human errors.
Evaluation of Endpoint: The endpoint of the titration is determined by observing a color change or a sudden drop in pH. However, it can be challenging to accurately judge the exact point of color change. One improvement that can be made is the use of a pH meter or an indicator with a sharper color change, which can provide more accurate results and minimize error in determining the endpoint.
Precision of Volumetric Glassware: The accuracy of the measurements relies on the precision of the volumetric glassware used, such as the burette and the pipette. It is important to ensure that the glassware is clean and calibrated properly. Regular calibration and maintenance of the glassware can help improve the accuracy of measurements.
Mixing and Equilibration: It is essential to ensure thorough mixing and equilibration of the solutions during the titration process. Incomplete mixing or insufficient equilibration can result in inaccurate results. To improve this, the solutions can be mixed more vigorously and allowed to equilibrate for a longer period of time before taking measurements.
In summary, while there are several sources of error that can influence the results of an acid-base titration lab, implementing the aforementioned improvements can help minimize these errors and enhance the reliability of the experiment. By reducing human error, improving the evaluation of the endpoint, ensuring precision of volumetric glassware, and enhancing mixing and equilibration, more accurate and consistent data can be obtained.
Q&A:
What are sources of error in scientific experiments?
Sources of error in scientific experiments can include human error, equipment malfunction, environmental factors, and sampling bias.
How can human error affect experimental results?
Human error can affect experimental results by introducing mistakes in data collection, recording, or analysis. This can lead to inaccurate or unreliable results.
What are some ways to minimize human error in experiments?
Some ways to minimize human error in experiments include providing proper training to experimenters, following standardized protocols, double-checking data, and using automated equipment when possible.
How does equipment malfunction contribute to errors in experiments?
Equipment malfunction can contribute to errors in experiments by producing inconsistent or unreliable measurements. This can affect the accuracy and precision of the data collected and analyzed.
What can be done to improve equipment reliability in experiments?
To improve equipment reliability in experiments, regular maintenance and calibration should be performed. Using high-quality equipment and properly storing and handling it can also help reduce the likelihood of malfunction.