
If you’re preparing for the AP Chemistry exam, you’re likely aware of the challenging free-response questions that test your knowledge and understanding of key concepts. The AP Chemistry 2021 Free-Response Questions were designed to assess your ability to apply your understanding of various chemistry topics in a real-world context.
In this article, we will provide you with answers to the free-response questions from the AP Chemistry 2021 exam. By reviewing the answers, you will gain a better understanding of the thought processes and approaches required to tackle these types of questions. Additionally, you’ll be able to identify any gaps in your knowledge and work on improving those areas before the actual exam.
Throughout the article, our expert chemistry tutors have broken down each question, explaining the reasoning and steps behind the correct answer. You’ll find detailed explanations and tips, ensuring that you not only understand the answers but also the underlying concepts. This will help you to effectively apply your knowledge to similar questions on the AP Chemistry exam.
Disclaimer: These answers are provided for educational purposes and may vary based on individual interpretation. It’s important to cross-reference with official AP Chemistry guidelines and consult with your instructor or tutor for any clarifications or additional guidance.
AP Chemistry 2021 Free-Response Questions Answers
The AP Chemistry 2021 free-response questions were released recently, and students across the country are eager to find the answers. These questions are a crucial part of the AP Chemistry exam, as they test students’ understanding of various concepts and their ability to apply them to real-world scenarios. In this article, we will discuss some of the possible answers to these free-response questions. It is important to note that these answers are not official and may vary depending on the individual student’s interpretation and approach.
One of the free-response questions in the AP Chemistry 2021 exam asked students to design an experiment to determine the concentration of a solute in a solution. One possible approach to this question is to use a spectrophotometer to measure the absorbance of the solution at a specific wavelength. By comparing the absorbance to a calibration curve, which relates absorbance to concentration, students can determine the concentration of the solute in the solution. It is important to control variables such as temperature, pH, and the concentration of other substances in the solution to ensure accurate results.
Another free-response question in the exam focused on equilibrium and Le Châtelier’s principle. Students were presented with a scenario where a reaction at equilibrium was disturbed by changing the concentration of one of the reactants. To determine the effect of this change on the equilibrium position, students can apply Le Châtelier’s principle. If the concentration of a reactant is increased, the equilibrium will shift in the direction that consumes that reactant, according to the principle. By considering the stoichiometry of the reaction and the equilibrium constant, students can determine the new equilibrium position and the effect of the disturbance.
In conclusion, the AP Chemistry 2021 free-response questions cover a range of topics and require students to apply their knowledge and problem-solving skills. The possible answers to these questions can vary depending on the individual student’s approach, but understanding key concepts and principles such as spectrophotometry and Le Châtelier’s principle can help in formulating a valid response. It is essential for students to practice with similar questions and seek guidance from their teachers to improve their performance in the AP Chemistry exam.
Description of the 2021 AP Chemistry Free-Response Questions
The 2021 AP Chemistry Free-Response Questions were designed to test students’ knowledge and understanding of various chemistry concepts and principles. These questions were part of the AP Chemistry exam, which is administered by the College Board to high school students who have completed a full year of advanced chemistry coursework.
Question 1: This question focused on the principles of thermodynamics and required students to calculate the enthalpy change for a chemical reaction using given data. Students were also asked to explain their reasoning and provide a clear mathematical solution.
Question 2: This question explored the concept of equilibrium and asked students to determine the equilibrium constant for a given reaction. Students were required to write the balanced chemical equation, set up an expression for the equilibrium constant, and calculate its value using provided data.
Question 3: This question delved into the topic of redox reactions and asked students to identify the species being reduced and oxidized in a chemical reaction. Students were also required to write separate half-reactions, balance them, and calculate the overall cell potential using standard reduction potentials.
Question 4: This question focused on the concept of kinetics and asked students to design an experiment to determine the rate law for a given reaction. Students were required to propose a suitable method, clearly outline the steps involved, and explain how the collected data would be used to determine the rate law.
Question 5: This question explored the principles of organic chemistry and asked students to identify the major organic product of a given reaction. Students were required to write the completed chemical equation, properly draw the structure of the product, and explain the reasoning behind their choice.
Overall, the 2021 AP Chemistry Free-Response Questions challenged students to apply their knowledge of various chemistry concepts in a problem-solving context. These questions required students to demonstrate their understanding of thermodynamics, equilibrium, redox reactions, kinetics, and organic chemistry principles. By successfully answering these questions, students showcased their ability to analyze and solve complex chemistry problems, which are essential skills for success in the field of chemistry and related disciplines.
Step-by-Step Solutions for AP Chemistry 2021 Free-Response Questions

Preparing for the AP Chemistry exam can be challenging, but understanding how to approach the free-response questions is crucial for success. The 2021 AP Chemistry exam included several free-response questions that required students to apply their knowledge and skills in various areas of the subject. In this article, we will provide step-by-step solutions for some of the key questions from the exam, helping you understand the correct approach and methodology.
Question 1: This question asked students to calculate the molar mass of a compound based on the given percent composition of its elements. To solve this problem, we can assume a 100 g sample of the compound and calculate the mass of each element based on the given percent composition. Then, we can divide the mass of each element by its molar mass to find the number of moles. Finally, by summing up the moles of each element, we can determine the molar mass of the compound.
Question 2: In this question, students were asked to calculate the pH of a weak acid solution. To solve this problem, we can start by writing the balanced chemical equation for the dissociation of the weak acid. Then, we can use the equilibrium constant expression to set up an equation representing the ionization of the acid and its conjugate base. By applying the equation and solving for the concentration of H+, we can use the pH formula to calculate the pH of the solution.
Question 3: This question involved determining the oxidation state of an element in a given compound. To solve this problem, we need to consider the oxidation states of the other elements in the compound and the overall charge of the compound. By assigning oxidation states to the other elements and applying the rules for assigning oxidation states, we can determine the oxidation state of the element in question.
These are just a few examples of the step-by-step solutions that can help you tackle the AP Chemistry free-response questions. Remember to review and understand the concepts and formulas relevant to each question before attempting to solve them. With practice and a solid understanding of the subject, you can improve your performance on the AP Chemistry exam.
Key Concepts and Topics Covered in AP Chemistry 2021 Free-Response Questions
AP Chemistry 2021 free-response questions covered a wide range of key concepts and topics in chemistry. These questions challenged students to apply their understanding of various principles and theories in order to solve complex problems and analyze experimental data.
One of the key concepts covered in the free-response questions was chemical equilibrium. Students were tested on their ability to calculate equilibrium constants, determine the direction of a reaction at specific conditions, and explain the factors that can affect the position of an equilibrium. Understanding Le Chatelier’s principle and its applications was crucial in answering these questions.
Another important area of focus in the free-response questions was thermodynamics. Students were asked to analyze enthalpy, entropy, and Gibbs free energy changes of chemical reactions. They had to apply the laws of thermodynamics to determine whether a reaction was spontaneous or non-spontaneous, and predict the effect of temperature changes on equilibrium constants and reaction rates.
The free-response questions also tested students’ knowledge of chemical kinetics. Students had to understand rate laws, reaction mechanisms, and factors that can affect the rate of a reaction. They were asked to calculate reaction rates, determine the order of a reaction, and propose a mechanism based on experimental data.
Overall, the AP Chemistry 2021 free-response questions emphasized the application of fundamental principles and concepts in chemistry. Students had to demonstrate their understanding of chemical equilibrium, thermodynamics, and kinetics, and their ability to analyze and interpret experimental data. These questions provided a comprehensive assessment of students’ knowledge and skills in various areas of chemistry.
Tips and Strategies for Answering AP Chemistry 2021 Free-Response Questions
Answering free-response questions on the AP Chemistry exam can be challenging, but with the right tips and strategies, you can maximize your score. Here are some key points to keep in mind as you approach the free-response section:
1. Understand the Question

Take the time to carefully read and understand each question before attempting to answer it. Pay attention to the specific instructions and any given data or diagrams. Make sure you know exactly what is being asked before formulating your response.
2. Show Your Work
In AP Chemistry, it’s not just about the final answer. The graders want to see your thought process and how you arrived at your solution. Show all relevant calculations, equations, and reasoning in your responses. Even if you make a mistake along the way, you can still earn partial credit for your approach.
3. Use Clear and Concise Language
When writing your answers, use clear and concise language to effectively convey your thoughts. Be sure to define any key terms and use proper scientific terminology when appropriate. Avoid excessive jargon or unnecessary details that may confuse the reader.
4. Practice Time Management
The free-response section of the AP Chemistry exam can be time-consuming. Allocate your time wisely, and don’t spend too much time on a single question. If you get stuck on a particular part, move on and come back to it later. Prioritize questions based on their point value and difficulty.
5. Use Proper Units and Significant Figures
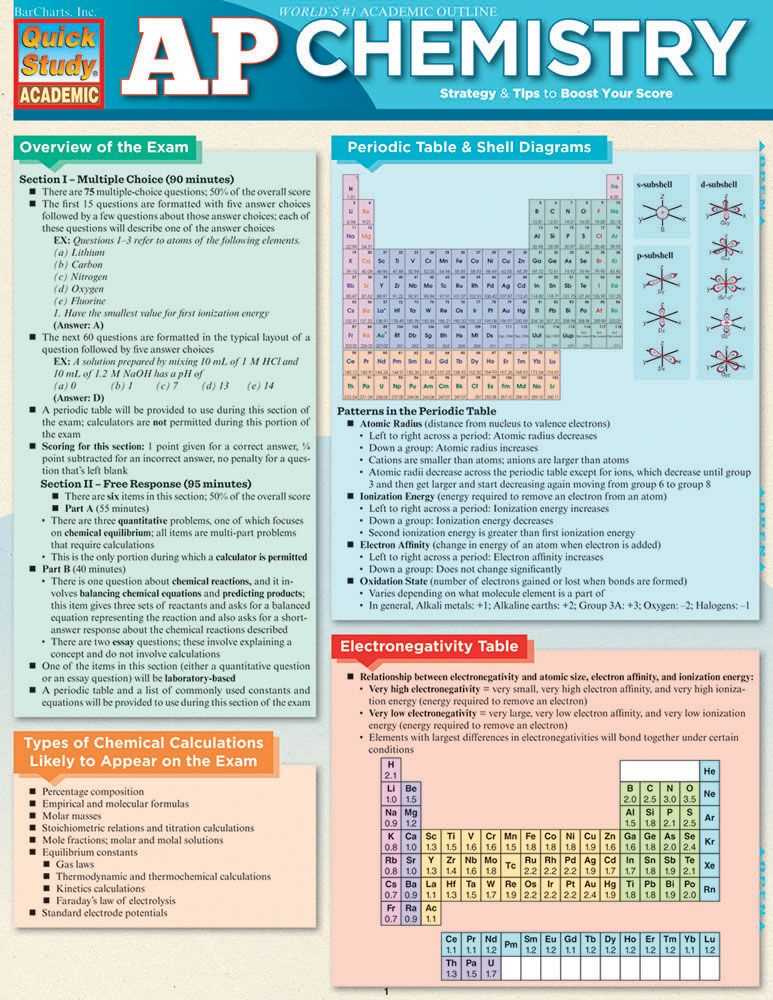
When providing numerical answers, always include the appropriate units and use the correct number of significant figures. This demonstrates your attention to detail and accuracy. Additionally, double-check your calculations and final answers to ensure they make sense and align with the given parameters.
By following these tips and strategies, you’ll be well-prepared to tackle the free-response questions on the AP Chemistry exam. Remember to practice past exams and review key concepts to further enhance your performance. Good luck!
Importance and Implications of the AP Chemistry 2021 Free-Response Questions
The AP Chemistry 2021 free-response questions have significant importance and implications for both educators and students. These questions serve as a tool to assess the students’ knowledge, skills, and understanding of the subject matter in a comprehensive way. By answering these questions, students can demonstrate their ability to apply their knowledge in real-world scenarios and showcase their problem-solving skills.
The importance of these free-response questions lies in their ability to:
- Evaluate conceptual understanding: The free-response questions are designed to test students’ understanding of fundamental concepts and principles in chemistry. It allows educators to gauge students’ comprehension and identify any gaps in their knowledge.
- Assess critical thinking skills: These questions require students to analyze and interpret data, make connections between different concepts, and apply their knowledge to solve complex problems. By doing so, students develop critical thinking skills that are crucial for success in higher education and beyond.
- Stimulate higher-level thinking: The free-response questions often present students with open-ended scenarios that require them to think creatively and independently. This encourages students to think beyond the textbook and apply their knowledge to unique situations.
- Provide feedback for improvement: The AP Chemistry free-response questions offer students an opportunity to receive feedback on their performance. By reviewing their answers and the provided scoring guidelines, students can identify areas of weakness and work to improve their understanding and skills.
Furthermore, the implications of the AP Chemistry 2021 free-response questions are:
- Preparation for college-level coursework: The challenging nature of these questions helps students to prepare for the rigor of college-level chemistry courses. By engaging with complex problems, students develop the necessary skills and confidence to succeed in higher education.
- Standardization of curriculum: The free-response questions create a standardized curriculum that guides educators in their teaching. Teachers can align their lessons and assessments with the content covered in these questions, ensuring consistency across different educational institutions.
- Evaluation of instructional effectiveness: The performance of students on the free-response questions can serve as an indicator of the effectiveness of the teaching methods and curriculum used by educators. This feedback allows educators to make adjustments and improvements to their instructional practices.
In conclusion, the AP Chemistry 2021 free-response questions play a crucial role in evaluating students’ knowledge and skills, promoting critical thinking and higher-level reasoning, and providing feedback for improvement. These questions also have implications for college preparation, curriculum standardization, and instructional effectiveness. By understanding the importance and implications of these questions, educators and students can make the most out of the AP Chemistry curriculum and enhance their overall learning experience.