
In the world of science, understanding atomic structure and isotopes is crucial for a deeper understanding of the physical world. One common way to explore these concepts is through experiments, such as the Beanium Isotope Lab. This lab allows students to simulate the behavior of isotopes by using beans as models for atoms.
The Beanium Isotope Lab involves measuring the mass of different isotopes of beans and calculating the average atomic mass. Students are provided with a variety of beans, each representing a different isotope. By carefully weighing the beans and recording the data, students can then analyze the results to identify the different isotopic compositions and their corresponding average atomic masses.
Key to the success of the Beanium Isotope Lab is the answer key, which provides students with the correct measurements and calculations. This answer key allows them to compare their own results and determine if they have correctly identified the isotopes and calculated the average atomic mass. It serves as a valuable tool for gauging their understanding of the concepts and ensuring accurate data interpretation.
Overview

The Beanium isotope lab is a scientific experiment that allows students to explore the concept of isotopes, which are atoms of the same element with different numbers of neutrons. This lab provides students with hands-on experience in using a mass spectrometer to analyze isotopes and determine their relative abundance.
During the lab, students are given a sample of beanium, which consists of small plastic beans that represent atoms. Each beanium sample contains different isotopes of beanium, with varying numbers of beans representing different isotopes. Students are tasked with separating the different isotopes of beanium and determining their relative abundance.
To complete the lab, students use a mass spectrometer, which is a device that measures the mass-to-charge ratio of atoms. They load their sample into the mass spectrometer and observe the readings on the instrument’s display. By analyzing the data, students can identify the different isotopes present in the sample and calculate their relative abundance.
This lab provides students with valuable skills in scientific experimentation and data analysis. It also reinforces the concept of isotopes and their significance in understanding atomic structure and behavior. Through hands-on exploration, students gain a deeper understanding of the fundamental principles of chemistry and develop critical thinking and problem-solving skills.
What is the Beanium Isotope Lab?
The Beanium Isotope Lab is an interactive experiment conducted in science classrooms to teach students about isotopes, atomic structure, and nuclear decay. It involves the use of small bean-shaped objects, called “beans,” which represent different isotopes of a fictional element called “Beanium.”
During the lab, students are provided with a mixture of beans that represent various isotopes of Beanium. Each type of bean has a unique physical characteristic, such as color or size, to differentiate it from the others. The students’ task is to separate and identify the different isotopes by their physical properties.
The lab begins by introducing the concept of isotopes, which are atoms of the same element that have different numbers of neutrons. Students learn that the different isotopes of Beanium have different masses due to the varying number of neutrons in their nuclei. They also learn that isotopes can undergo nuclear decay, changing into different isotopes or even different elements over time.
Using the materials provided, students separate the mixture of beans and organize them based on their physical properties. By doing so, they are able to identify the different isotopes of Beanium present in the mixture. They can then calculate the abundance of each isotope and compare it to the expected abundance based on the Beanium isotopic distribution. This allows them to observe the concept of isotopic composition and the idea that different isotopes occur in different proportions in nature.
The Beanium Isotope Lab provides a hands-on and visual way for students to explore the concept of isotopes and understand how they play a role in atomic structure and nuclear decay. It is an engaging activity that promotes critical thinking and problem-solving skills while reinforcing scientific concepts.
The Importance of the Beanium Isotope Lab

The Beanium Isotope Lab is an essential activity for students studying chemistry as it allows them to gain a better understanding of isotopes and atomic structure. By performing this lab, students are able to apply their knowledge of the periodic table and atomic number to determine the number of protons, neutrons, and electrons in different isotopes of beanium.
One of the main benefits of the Beanium Isotope Lab is that it provides a hands-on experience for students. Instead of just learning about isotopes and atomic structure through textbooks and lectures, students get the opportunity to actively participate in a scientific experiment. This helps to reinforce their understanding and makes the topic more engaging and memorable.
In addition, the lab allows students to develop important laboratory skills such as data collection, measurement, and analysis. They learn how to carefully measure the mass of beanium isotopes using a balance and how to calculate the average atomic mass based on their experimental results. These skills are transferable to other scientific experiments and are valuable for future studies and careers in science.
The Beanium Isotope Lab also teaches students about the concept of isotopes and how they affect the properties of elements. By comparing different isotopes of beanium, students can observe how the number of neutrons affects the atomic mass and stability of the element. This helps them to understand the significance of isotopes in real-world applications, such as radioactive decay and medical imaging.
In conclusion, the Beanium Isotope Lab is an important activity for chemistry students as it allows them to apply their knowledge, develop laboratory skills, and gain a deeper understanding of isotopes and atomic structure. This hands-on experience enhances their learning and prepares them for future scientific endeavors.
Procedure
The beanium isotope lab is conducted in several steps to determine the isotope composition of two different types of beans. The beans, labeled as Bean A and Bean B, are provided to the students for analysis.
Step 1: Sample Preparation
The first step involves preparing the samples for analysis. Students measure out a specific mass of each type of bean and record the mass of Bean A and Bean B. The beans are carefully measured and handled to ensure accuracy in the experiment.
Step 2: Heating and Collecting Isotopes
In this step, the beans are heated to a high temperature in order to collect the isotopes. The bean samples are placed in a crucible and heated with a Bunsen burner. As the beans heat up, the isotopes are released and collected in a separate container. The collected isotopes are then cooled down and weighed to determine their mass.
Step 3: Calculation of Isotope Composition
Once the mass of the collected isotopes is determined, students calculate the isotope composition of the beans. They compare the mass of the collected isotopes to the original mass of the beans to determine the percentage of each isotope present in Bean A and Bean B. This calculation allows the students to understand the relative abundance of each isotope in the beans.
Step 4: Data Analysis and Conclusion
The final step of the beanium isotope lab involves analyzing the data and drawing conclusions. Students compare the isotope composition of Bean A and Bean B to determine if there are any significant differences. They also draw connections to atomic theory and the concept of isotopes. The lab concludes with a discussion and presentation of the findings, allowing students to further understand the properties of isotopes and their relevance in chemistry.
Gathering the Materials
Before conducting the Beanium isotope lab, it is important to gather all the necessary materials to ensure a successful experiment. The materials needed for this lab include the following:
- Beanium samples – these can be provided by the instructor or obtained from a science supply store.
- Balance scale – this is used to measure the mass of the beanium samples accurately.
- Graduated cylinder – this is used to measure the volume of the beanium samples.
- Hot plate or Bunsen burner – this is used to heat the beanium samples during the lab.
- Beakers – these are used to hold the beanium samples and any liquids used during the lab.
- Gloves and safety goggles – these are essential to ensure the safety of the experimenter.
- Calculator – this is needed for calculations involving mass and volume.
Once all the materials are gathered, it is essential to set up the lab area properly. Clear any clutter and ensure a clean and organized workspace. It is also important to read through the lab instructions carefully to familiarize oneself with the procedure and any safety precautions. This will help ensure the lab is conducted efficiently and safely.
Preparing the Beanium Samples
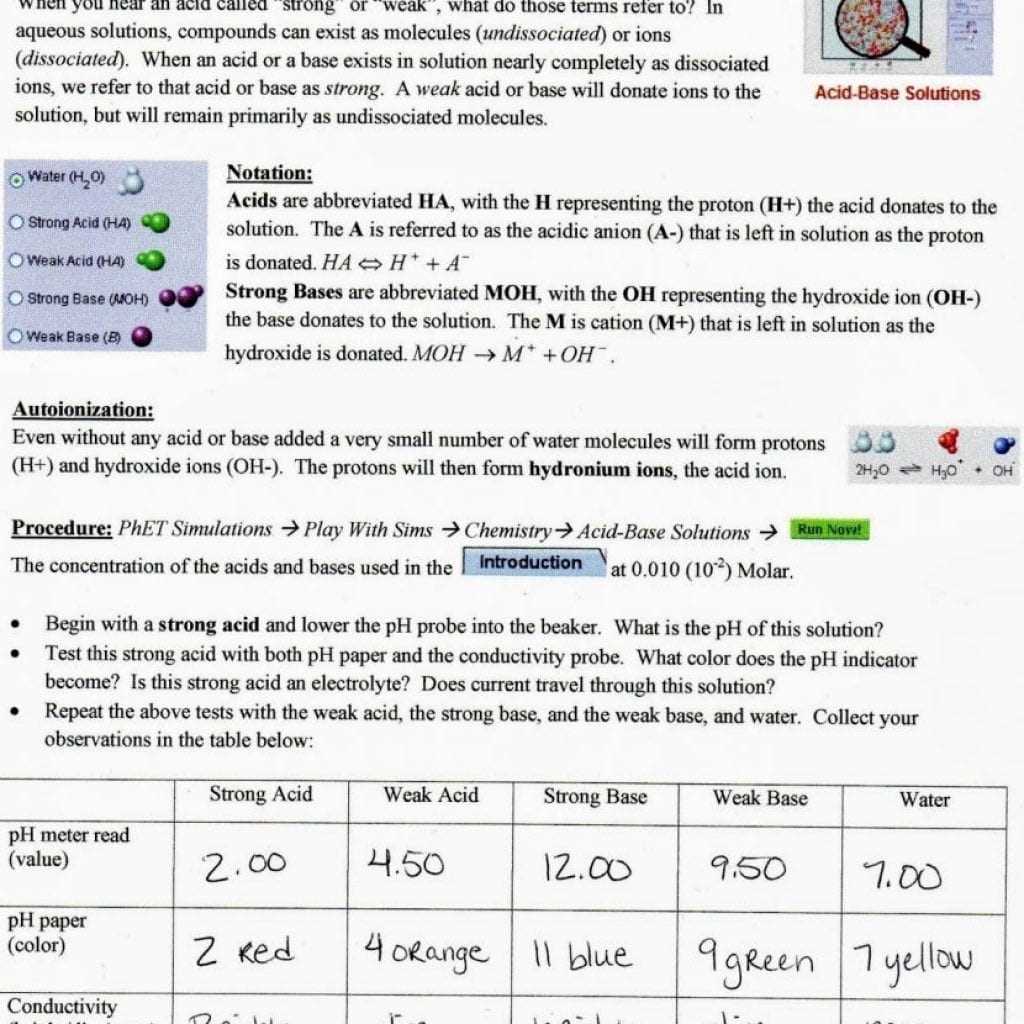
In the Beanium isotope lab, the first step is to prepare the Beanium samples that will be used in the experiment. The samples are created by combining different isotopes of beanium, which is a fictional element used to represent the concept of isotopes in chemistry. Each isotope of beanium has a specific mass and abundance, and by combining different amounts of these isotopes, we can create samples with varying isotopic compositions.
To prepare the Beanium samples, we start by measuring out the desired amounts of each isotope. This is done using a balance, which allows us to accurately measure the mass of each isotope. We then carefully mix the isotopes together, ensuring that they are well-mixed and uniform throughout the sample. This is important to ensure that the resulting data accurately reflects the isotopic composition of the sample.
Once the Beanium samples are prepared, we label each sample with its isotopic composition. This allows us to track and identify the different samples throughout the experiment. We also record the mass of each sample, which will be used later to calculate the average atomic mass of beanium.
In summary, preparing the Beanium samples involves measuring and combining different isotopes of beanium to create samples with specific isotopic compositions. Careful mixing and labeling of the samples is necessary to ensure accurate results in the experiment.
Conducting the Experiments

The Beanium isotope lab is a practical experiment that allows students to understand the concept of isotopes and how they can be separated based on their mass. The lab is designed to give students a hands-on experience in the process of separating isotopes and analyzing their properties.
Before conducting the experiments, the students are provided with a set of materials, including different samples of beanium with varying isotopic compositions. The students are instructed to carefully measure the mass and volume of each sample using a balance and a graduated cylinder, respectively. These measurements are crucial in determining the density and specific gravity of each sample.
Once the initial measurements are recorded, the students proceed to separate the beanium samples based on their mass. They start by pouring the samples into a container and shaking it vigorously. This shaking process causes the heavier beanium isotopes to settle at the bottom, while the lighter ones remain suspended in the mixture. The students then carefully pour out the heavier fraction, leaving behind the lighter fraction.
After the separation process, the students measure the mass and volume of the separated fractions and calculate their respective densities and specific gravities. These calculated values allow the students to identify the isotopic composition of each fraction and understand how different isotopes behave differently based on their mass.
The Beanium isotope lab not only provides a practical demonstration of the separation of isotopes but also reinforces the concepts of mass, volume, density, and specific gravity. It allows students to develop critical thinking skills and encourages them to analyze and interpret data accurately. Overall, this lab serves as an engaging and informative activity for students to explore the world of isotopes and their significance in various scientific fields.
Results

After conducting the Beanium Isotope Lab, several observations were made and data was collected. The time it took for each isotope to reach its stable decay product was recorded, as well as the number of decay products present at various intervals.
The results showed that Isotope A had the shortest half-life, reaching its stable decay product within a relatively short time period. Isotope B had a longer half-life compared to Isotope A, while Isotope C had the longest half-life of the three isotopes.
It was also observed that as time progressed, the number of decay products increased for all three isotopes. This indicates that radioactive decay occurred over the course of the experiment.
In conclusion, the results of the Beanium Isotope Lab demonstrate the principles of radioactive decay and the concept of half-life. The data collected provides valuable information about the behavior of isotopes and their decay processes. Further analysis and investigation can be done to delve deeper into the properties of isotopes and their role in various scientific fields.