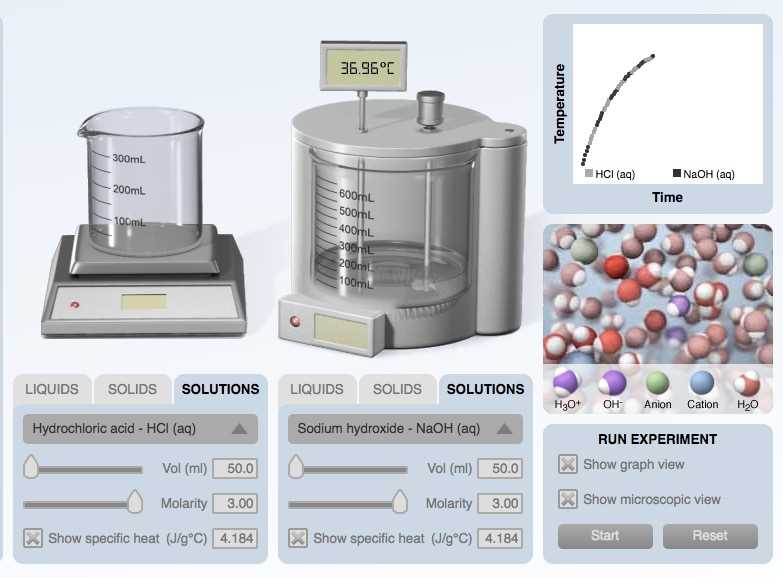
In the world of science and chemistry, calorimetry is a fundamental concept that allows us to measure and understand heat transfer. The Calorimetry Lab Gizmo is an innovative tool that brings this concept to life, allowing students and learners of all ages to explore and experiment with the principles of calorimetry in a virtual setting.
But what is calorimetry, and why is it so important? Calorimetry is the science of measuring the amount of heat transferred during a chemical reaction or a physical change. By understanding and quantifying heat transfer, scientists can better grasp the energy changes involved in various processes, whether it’s combustion, phase changes, or even biochemical reactions.
The Calorimetry Lab Gizmo answers the call for a practical and hands-on approach to learning about calorimetry. With this interactive simulation, users can set up their own virtual experiments, measuring the heat flow between substances, determining specific heat capacities, and even calculating the unknown heat of a reaction. This immersive experience fosters a deeper understanding of the concepts at play, making it an invaluable educational tool for students and teachers alike.
So, whether you’re a student looking to ace your next chemistry test or a teacher searching for engaging and effective teaching resources, the Calorimetry Lab Gizmo answers the need for a dynamic and comprehensive learning experience. Step into the world of calorimetry and unlock the secrets of heat transfer with this powerful educational tool.
What is Calorimetry Lab Gizmo?
Calorimetry Lab Gizmo is an interactive online simulation tool that allows students to explore the concepts of calorimetry in a virtual laboratory setting. Calorimetry is the scientific measurement of the heat of reactions and changes in heat energy. This Gizmo provides a hands-on experience for students to study heat transfer, specific heat capacity, and how different factors affect thermal energy.
The Calorimetry Lab Gizmo allows students to conduct experiments by manipulating various parameters such as initial temperature, mass, and specific heat capacity of substances. It provides a realistic virtual laboratory environment with tools to measure heat changes and record data. Students can observe how thermal energy is transferred between substances, calculate specific heat capacity, and analyze the effects of different variables on temperature changes.
The Gizmo also offers a detailed explanation of the underlying principles and equations involved in calorimetry. Students can read about the theory behind the experiments they are conducting and learn about the significance of calorimetry in various scientific fields, such as chemistry, physics, and biology. This interactive tool enhances the understanding of calorimetry concepts through hands-on experimentation and exploration, making it a valuable resource for both teachers and students.
How to Use Calorimetry Lab Gizmo
The Calorimetry Lab Gizmo is a useful tool that allows you to study the transfer of heat energy during chemical reactions. This interactive simulation provides a virtual laboratory where you can conduct experiments and explore various aspects of calorimetry. Here are some steps to help you get started:
1. Setting up the experiment:
Begin by selecting the chemicals you want to use from the available options. You can choose from a variety of substances, such as water, ethanol, or a specific metal. Set the initial conditions, including the mass and temperature of each substance.
2. Conducting the experiment:
Once you have set up the initial conditions, you can start the experiment. The Gizmo will simulate the mixing of the substances and measure the change in temperature. You can also adjust other parameters, such as the quantity of substances or the energy provided to the system.
3. Collecting data:
During the experiment, the Gizmo will display real-time data, including the change in temperature and the heat flow. You can record these measurements and use them to analyze the heat transfer and the nature of the reaction. You can also compare the results of different experiments to draw conclusions and make predictions.
4. Analyzing the results:
After completing the experiment, you can analyze the data and observe any trends or patterns. You can use the provided tools, such as graphs or calculations, to interpret the results and draw conclusions about the energy exchange and the efficiency of the reaction. You can also compare your findings with known scientific principles and theories.
The Calorimetry Lab Gizmo is a powerful learning tool that allows you to explore the fundamental principles of calorimetry in a virtual laboratory setting. By conducting experiments, collecting data, and analyzing the results, you can develop a deeper understanding of heat transfer and its role in chemical reactions.
Step-by-Step Guide to Using Calorimetry Lab Gizmo
Calorimetry Lab Gizmo is an online simulation tool that allows you to explore the concepts of calorimetry and the measurement of heat transfer. It is an interactive and educational tool that helps students understand the principles and calculations involved in calorimetry experiments.
To get started with the Calorimetry Lab Gizmo, follow these step-by-step instructions:
1. Access the Gizmo
Visit the website or platform where the Calorimetry Lab Gizmo is available. Log in with your credentials, if required.
2. Start a New Experiment
Once you have accessed the Gizmo, you will see the interface and options available. Click on the “Start New Experiment” button to begin.
3. Select the Materials
In the Gizmo, you will be presented with a variety of materials that you can use for your experiment. Choose the objects or substances that you want to use and drag them into the appropriate places in the Gizmo.
4. Set Initial Conditions
Set the initial conditions of the experiment, such as the initial temperature of the objects or substances and the amount of each material being used.
5. Start the Experiment
When all the materials and initial conditions are set, click on the “Start” or “Begin Experiment” button to initiate the experiment.
6. Observe the Experiment
Observe and record the changes in temperature and the heat transfer between the objects or substances. Take note of any observations or patterns you notice during the experiment.
7. Analyze the Data
After the experiment, analyze the data you gathered and perform any necessary calculations or calculations provided by the Gizmo to determine the quantity of heat transferred during the experiment.
8. Repeat and Explore
Once you have completed one experiment, feel free to repeat it with different materials or conditions to further explore the principles of calorimetry. Play around with the variables and observe the effects on the heat transfer.
By following these steps, you can effectively use the Calorimetry Lab Gizmo to learn and understand the concepts of calorimetry and the measurement of heat transfer.
What Are the Answers for Calorimetry Lab Gizmo?
In the Calorimetry Lab Gizmo, students use a calorimeter to determine the amount of heat energy transferred in various chemical reactions. While it is important for students to actively engage in the experiment and explore the concepts themselves, here are some general answers that may be helpful:
1. Which substance has the highest specific heat capacity?
The substance with the highest specific heat capacity is water. This is because water has a high capacity to store and transfer heat energy, making it an effective coolant.
2. What is the heat capacity of the calorimeter?
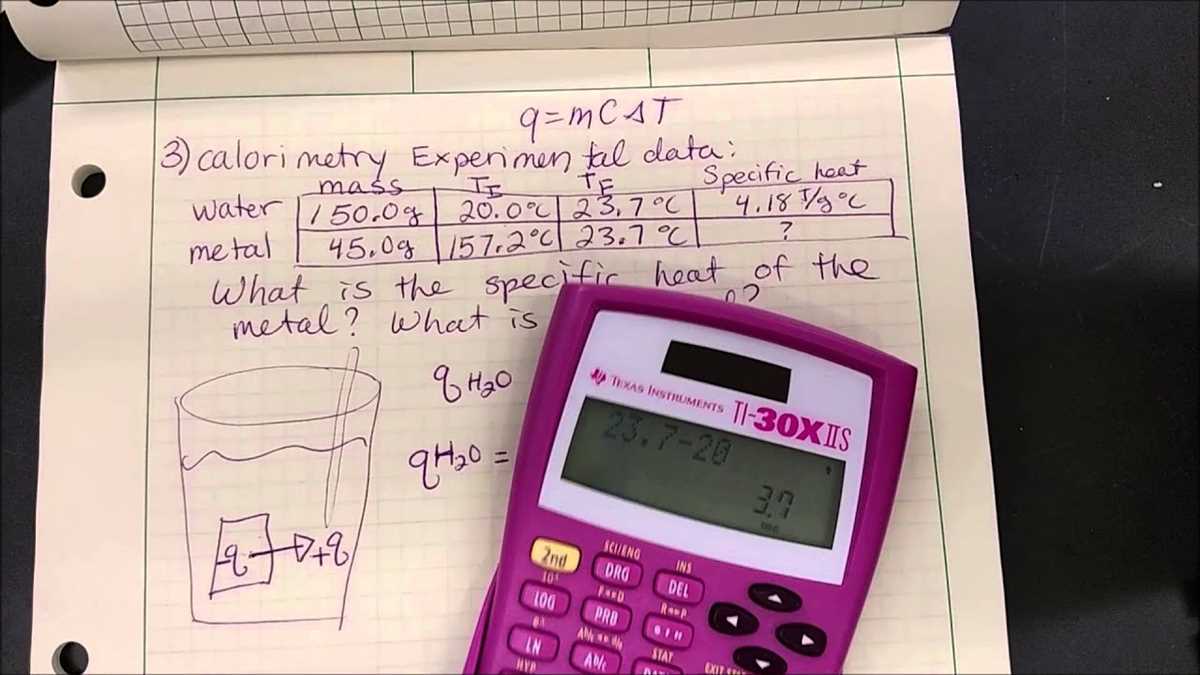
The heat capacity of the calorimeter can be determined by performing a calibration experiment. By adding a known amount of heat to the calorimeter and measuring the resulting temperature change, the heat capacity can be calculated using the equation Q = mcΔT, where Q is the heat energy, m is the mass, c is the specific heat capacity, and ΔT is the temperature change.
3. What is the heat of reaction for the given chemical reaction?
The heat of reaction can be determined by measuring the temperature change of the solutions before and after the reaction occurs. The heat released or absorbed during the reaction can be calculated using the equation Q = mcΔT, where Q is the heat energy, m is the mass, c is the specific heat capacity, and ΔT is the temperature change.
- These answers provide a general overview of the concepts and calculations involved in the Calorimetry Lab Gizmo. It is important for students to actively participate in the experiment and analyze their own data to fully understand the principles of calorimetry and heat transfer. The Gizmo provides a hands-on learning experience that allows students to explore these concepts in a practical and interactive way.
Commonly Asked Questions and Answers for Calorimetry Lab Gizmo
Q: What is the purpose of the Calorimetry Lab Gizmo?
The purpose of the Calorimetry Lab Gizmo is to provide students with a virtual laboratory experience where they can explore the concept of calorimetry and learn how to measure the heat of various substances. Through hands-on experimentation, students can understand the principles of calorimetry and gain a deeper understanding of the transfer of heat energy.
Q: How does the Calorimetry Lab Gizmo work?
The Calorimetry Lab Gizmo simulates a virtual laboratory setting where students can conduct experiments using a calorimeter. They can choose different substances, measure their initial and final temperatures, and calculate the heat change using the formula Q = mcΔT, where Q is the heat energy, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature. The Gizmo provides real-time feedback and allows students to observe the effects of different variables on the heat transfer process.
Commonly asked questions:
- Q: How can I change the substance in the calorimeter?
- Q: Can I change the mass of the substance?
- Q: How can I calculate the heat change using the Gizmo?
A: To change the substance in the calorimeter, you can click on the “Substance” drop-down menu and select a different substance from the available options. This will change the specific heat capacity of the substance and allow you to observe how different substances affect the heat transfer process.
A: Yes, you can change the mass of the substance by adjusting the slider next to the “Mass” label. By increasing or decreasing the mass, you can observe how it affects the amount of heat transferred and the final temperature of the substance.
A: The Gizmo automatically calculates the heat change for you based on the initial and final temperatures, mass, and specific heat capacity of the substance. The calculated heat change is displayed in the “Heat change (Q)” field. If you want to manually calculate the heat change, you can use the formula Q = mcΔT and input the values in the appropriate fields.
Applications and Benefits of Using Calorimetry Lab Gizmo
Calorimetry Lab Gizmo is a valuable tool for conducting experiments and studying the science of heat transfer. It provides a virtual lab environment where students can explore and understand the principles of calorimetry and its applications in various fields. The gizmo allows users to measure the heat energy involved in chemical reactions, the specific heat capacity of different substances, and even calculate the amount of heat exchanged in everyday scenarios.
One of the main benefits of using the Calorimetry Lab Gizmo is its accessibility and ease of use. Unlike traditional laboratory experiments, the gizmo eliminates the need for physical equipment and supplies, making it cost-effective and convenient for both students and educators. Additionally, the virtual nature of the gizmo allows for unlimited experimentation and data collection without the limitations of time and resources.
The Calorimetry Lab Gizmo also promotes critical thinking and problem-solving skills. Students are encouraged to analyze data, make observations, and draw conclusions based on their experiments. They can manipulate variables, such as the mass and temperature of substances, and observe the corresponding changes in heat energy. This hands-on approach to learning enhances students’ understanding of scientific concepts and fosters a deeper appreciation for the subject.
Furthermore, the Calorimetry Lab Gizmo offers real-world applications that demonstrate the relevance of calorimetry in various industries. Students can explore how calorimetry is used in the food industry to determine the calorie content of different foods and beverages. They can also investigate its applications in environmental science, such as measuring the heat energy released during combustion reactions or studying the impact of climate change on the Earth’s temperature.
In conclusion, the Calorimetry Lab Gizmo is a valuable educational tool that enhances the learning experience in the field of calorimetry. Its accessibility, ease of use, and real-world applications make it a useful resource for students and educators alike. By utilizing this virtual lab environment, students can develop their scientific skills, deepen their understanding of heat transfer, and explore the wide range of applications that calorimetry has in various industries.
Exploring the Practical Uses and Advantages of Calorimetry Lab Gizmo
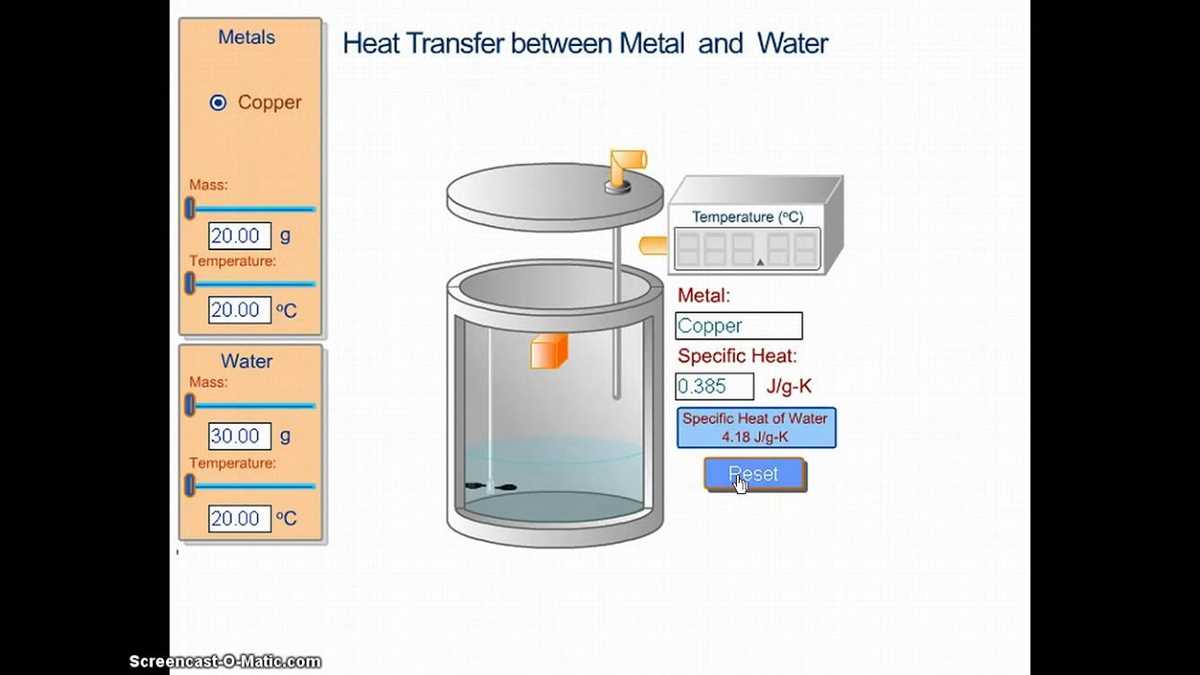
Calorimetry Lab Gizmo is a powerful tool that allows students to explore the science of calorimetry in a hands-on and interactive way. This virtual laboratory provides a realistic simulation environment where students can conduct experiments, collect data, and analyze their results. The practical uses and advantages of Calorimetry Lab Gizmo can be seen in various educational settings.
- Enhancing Learning: Calorimetry Lab Gizmo offers a unique opportunity for students to engage with the subject matter in a more interactive and immersive way. By manipulating variables, conducting experiments, and observing the effects of different factors on the system, students can develop a deeper understanding of the principles of calorimetry.
- Promoting Critical Thinking: The Gizmo encourages students to think critically and analyze data. Through the process of hypothesis testing, students can develop their problem-solving skills and learn to draw logical conclusions based on their observations. This fosters the development of analytical thinking and scientific reasoning.
- Experimentation without Constraints: Calorimetry Lab Gizmo eliminates the practical limitations of conducting experiments in a traditional laboratory setting. Students can conduct multiple trials, explore different scenarios, and manipulate variables easily, allowing them to experiment and learn from their mistakes without the fear of wasting resources or causing harm.
- Time and Cost-Efficient: The virtual nature of Calorimetry Lab Gizmo eliminates the need for physical materials and equipment, making it a cost-effective solution for educational institutions. Additionally, students can access the Gizmo from any location, allowing for flexible and independent learning.
- Real-World Application: Calorimetry Lab Gizmo provides students with a platform to apply their knowledge and skills to real-world scenarios. By simulating practical situations, such as measuring the energy content of food or determining the specific heat capacity of substances, students can gain insights into the practical applications of calorimetry in various fields like chemistry, physics, and biology.
In conclusion, Calorimetry Lab Gizmo is an invaluable educational tool that enhances learning, promotes critical thinking, allows for experimentation without constraints, is time and cost-efficient, and provides real-world applications. By using this virtual laboratory, students can develop a strong foundation in calorimetry principles and gain the necessary skills to succeed in scientific disciplines.