Welcome to the answer key for Chapter 4 Review on the Arrangement of Electrons in Atoms. In this chapter, we delve into the fascinating world of quantum mechanics and explore how electrons are organized within an atom. Understanding the arrangement of electrons is crucial for comprehending the behavior and properties of elements.
The key focus of this chapter is the quantum mechanical model of the atom, which provides a more accurate description of the electron arrangement compared to earlier models. We will explore the concept of energy levels and sublevels, as well as the importance of electron configuration and periodic trends.
Through this review, you will have the opportunity to test your understanding of electron arrangement by practicing with various examples and exercises. The answer key will provide you with detailed explanations and solutions to help you solidify your knowledge and prepare for any upcoming quizzes or exams.
So, let’s dive into the review and sharpen our understanding of the arrangement of electrons in atoms. This knowledge will not only deepen our understanding of the atomic world but also pave the way for a deeper appreciation of the periodic table and the elements it encompasses. Get ready to unravel the mysteries of electron arrangement!
Chapter 4 Review Arrangement of Electrons in Atoms Answer Key
The arrangement of electrons in atoms is a fundamental concept in chemistry. Understanding how electrons are distributed within an atom helps us to predict its chemical behavior and properties. In this chapter review, we will explore the key concepts related to the arrangement of electrons in atoms.
One of the key concepts discussed in this chapter is the electron configuration of an atom. The electron configuration describes the distribution of electrons in the different energy levels or orbitals of an atom. It is written using a notation that indicates the energy level, orbital type, and the number of electrons in each orbital. By understanding the electron configuration, we can determine the stability and reactivity of an atom.
Another important concept covered in this chapter is the periodic table and its relationship to electron configuration. The periodic table is organized based on the increasing atomic number, which corresponds to the number of protons in an atom. The arrangement of elements in the periodic table reflects the filling order of electron orbitals. By understanding the periodic table and electron configuration, we can predict the location and properties of different elements.
Overall, this chapter review provides a comprehensive understanding of the arrangement of electrons in atoms. By mastering the concepts of electron configuration and the periodic table, we can gain insights into the behavior and properties of atoms, which is essential in understanding chemical reactions and the composition of matter.
Overview of Electron Configuration
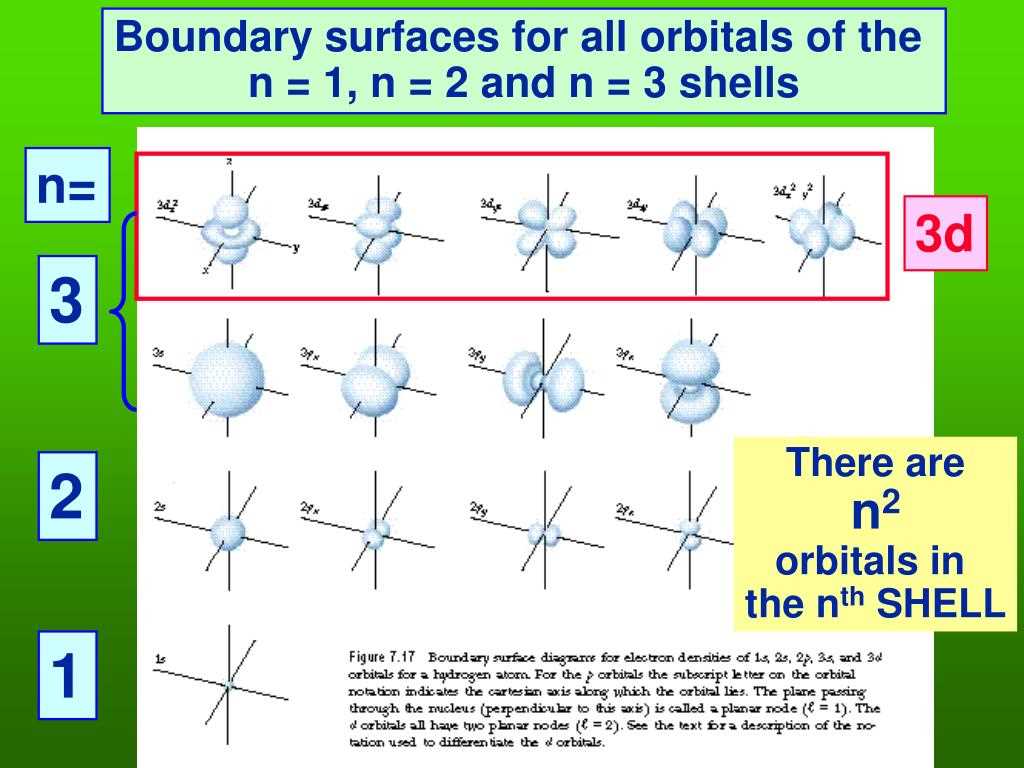
In chemistry, electron configuration refers to the arrangement of electrons in an atom, molecule, or ion. Understanding electron configuration is essential for understanding the chemical behavior and properties of an element. It provides insight into how electrons are distributed among different energy levels and orbitals within an atom.
Electron configuration follows a specific set of rules and principles. The Aufbau principle states that electrons occupy the lowest energy levels and orbitals first before filling higher energy levels. The Pauli exclusion principle dictates that no two electrons in an atom can have the same set of quantum numbers, meaning that each electron must have its own unique combination of principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s).
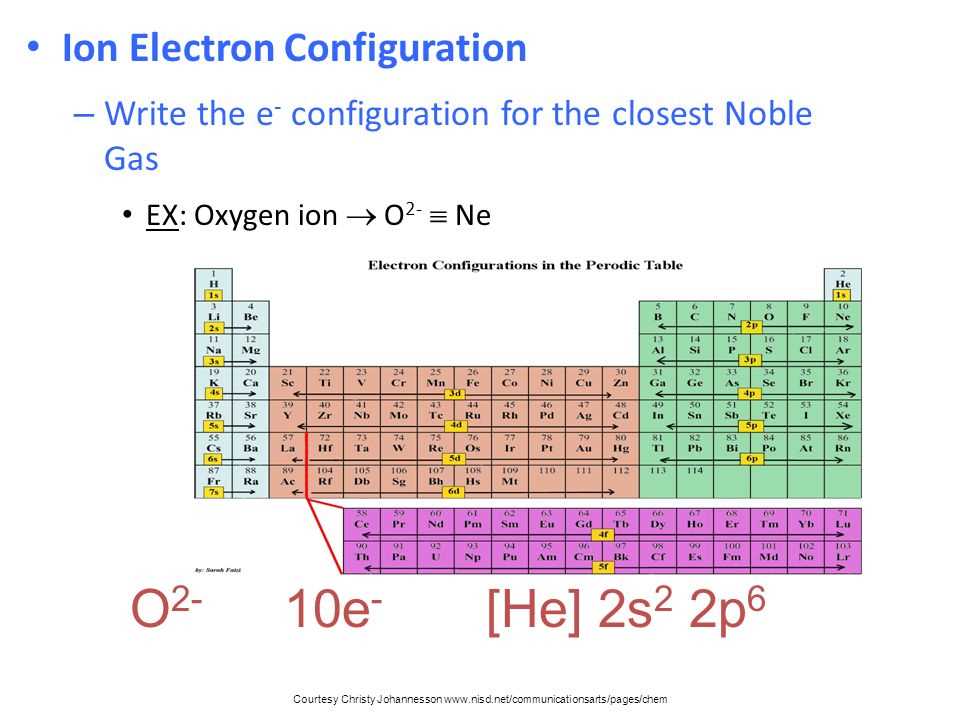
The electron configuration of an atom is typically represented using a shorthand notation, such as the noble gas notation. This notation uses the symbol of the noble gas that comes before the element being described, followed by the electron configuration of the remaining electrons. For example, the electron configuration of oxygen (O) can be represented as [He] 2s^2 2p^4, indicating that it has two electrons in the 2s orbital and four electrons in the 2p orbital.
Understanding electron configuration is important because it helps predict an element’s chemical reactivity and behavior. It provides information about an atom’s stability, valence electrons, and ability to form chemical bonds. Electron configuration also plays a crucial role in determining an element’s position in the periodic table and its properties.
In summary, electron configuration is the arrangement of electrons in an atom, molecule, or ion. It follows specific rules and principles and is represented using shorthand notation. Understanding electron configuration is essential for understanding an element’s chemical behavior and properties.
Rules for Filling Electron Orbitals
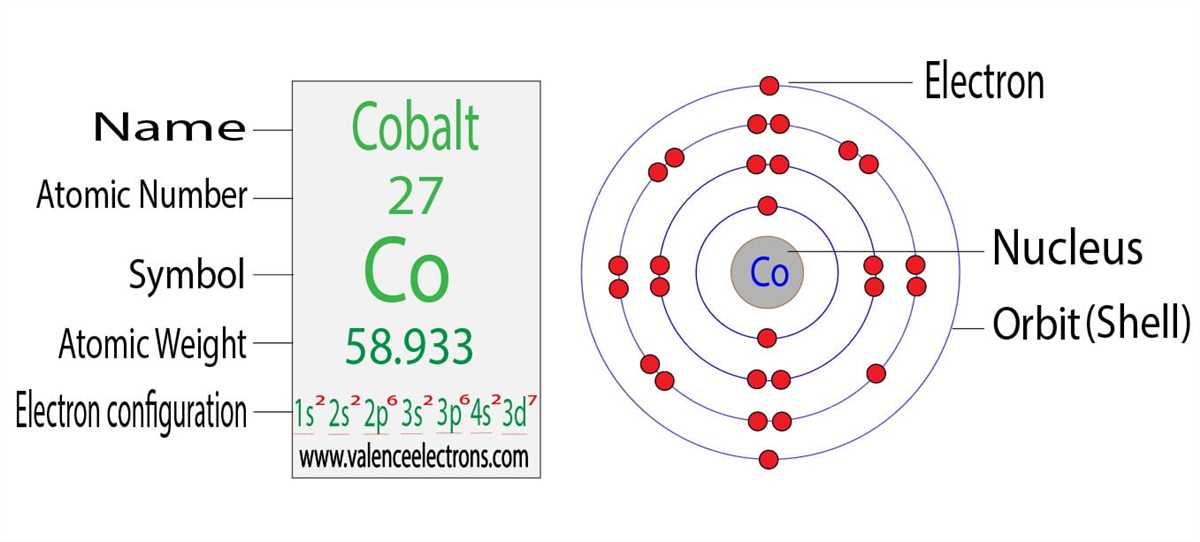
When it comes to filling electron orbitals, there are specific rules that dictate the arrangement of electrons in an atom. These rules are based on the principles of quantum mechanics and help to determine the energy levels and distribution of electrons within an atom.
1. Aufbau principle: This rule states that electrons fill the lowest energy orbitals first before moving on to higher energy levels. The orbitals are filled in a specific order, starting with the 1s orbital, followed by the 2s, 2p, 3s, and so on. Electrons will always occupy the lowest energy orbital available.
2. Hund’s rule: If there are multiple orbitals with the same energy, electrons will first fill each orbital with one spin before pairing up. This means that if there are three orbitals in a sublevel, each with one electron, they will all have the same spin before any electrons begin to pair up.
3. Pauli exclusion principle: This principle states that no two electrons within an atom can have the same set of four quantum numbers. Therefore, each electron must have a unique combination of spin and orbital occupation.
By following these rules, scientists can determine the electron configuration of an atom, which describes the arrangement of electrons in its orbitals. This information is crucial in understanding the chemical behavior and properties of elements.
- Example: The electron configuration of oxygen (O) is 1s^2 2s^2 2p^4. This means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and four electrons in the 2p orbital.
Understanding the rules for filling electron orbitals allows scientists to predict the behavior of different elements and their interactions with other particles. It provides a foundation for exploring the properties and behaviors of atoms and molecules, contributing to advancements in various fields of science and technology.
Valence Electrons and Periodic Table
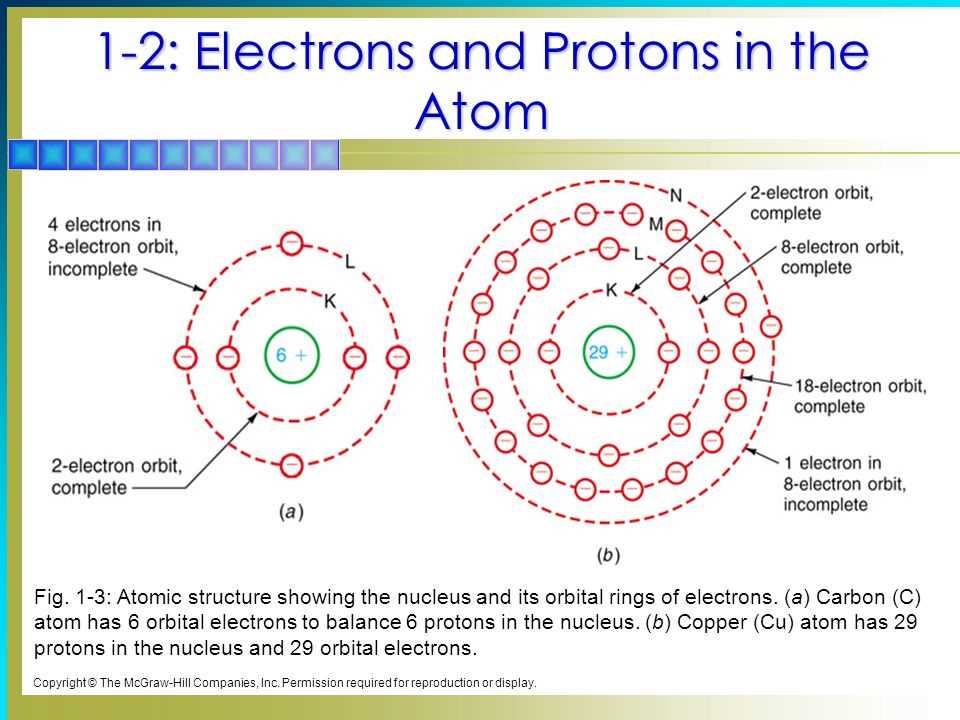
Valence electrons are the electrons located in the outermost energy level of an atom. These electrons play a crucial role in determining an atom’s chemical behavior and its ability to form bonds with other atoms. The number of valence electrons an atom has can be determined by its position in the periodic table.
The periodic table is a useful tool for understanding the arrangement of electrons in atoms. It organizes the elements based on their atomic number and electron configuration. The periodic table is divided into periods, which are horizontal rows, and groups, which are vertical columns. The group number corresponds to the number of valence electrons an atom of that element has.
In general, atoms in the same group have similar chemical properties because they have the same number of valence electrons. For example, elements in Group 1 all have 1 valence electron and are highly reactive metals. Elements in Group 18, also known as the noble gases, have full valence electron shells and are unreactive.
The arrangement of electrons in atoms can also be predicted using the periodic table. For example, elements in Group 1 will typically lose their valence electron and form a positive ion with a charge of +1. Elements in Group 17 will typically gain one electron to fill their valence shell and form a negative ion with a charge of -1.
Understanding the concept of valence electrons and their placement in the periodic table is essential in predicting the chemical behavior and reactivity of elements. It allows scientists to make predictions about how atoms will interact and form compounds. The periodic table provides a visual representation of this information, making it a valuable tool in the study of chemistry.
Electron Configurations and Energy Levels
Electron configurations describe the arrangement of electrons in an atom. The distribution of these electrons is based on their energy levels. The energy levels of an atom are represented by different shells or orbitals, which are designated as s, p, d, and f. Each energy level can hold a specific number of electrons, with the s orbital holding 2 electrons, the p orbital holding 6 electrons, the d orbital holding 10 electrons, and the f orbital holding 14 electrons.
The arrangement of electrons in an atom is determined by the Aufbau principle, the Pauli exclusion principle, and Hund’s rule. The Aufbau principle states that electrons fill the lowest energy levels first before occupying higher energy levels. The Pauli exclusion principle states that each electron in an atom must have a unique set of quantum numbers, and therefore, no two electrons can occupy the same orbital with the same spin. Hund’s rule states that electrons will fill orbitals of the same energy level with parallel spins before pairing up.
Understanding electron configurations and energy levels is crucial in predicting an atom’s chemical behavior and reactivity. By knowing the arrangement of electrons in an atom, scientists can determine its valence electrons, which are responsible for an atom’s chemical properties and ability to bond with other atoms.
In summary, electron configurations describe the arrangement of electrons in an atom based on their energy levels. Each energy level can hold a specific number of electrons, and the distribution of these electrons is determined by the Aufbau principle, the Pauli exclusion principle, and Hund’s rule. Understanding electron configurations and energy levels is essential in understanding an atom’s chemical behavior and reactivity.
Noble Gas Configuration and Stability
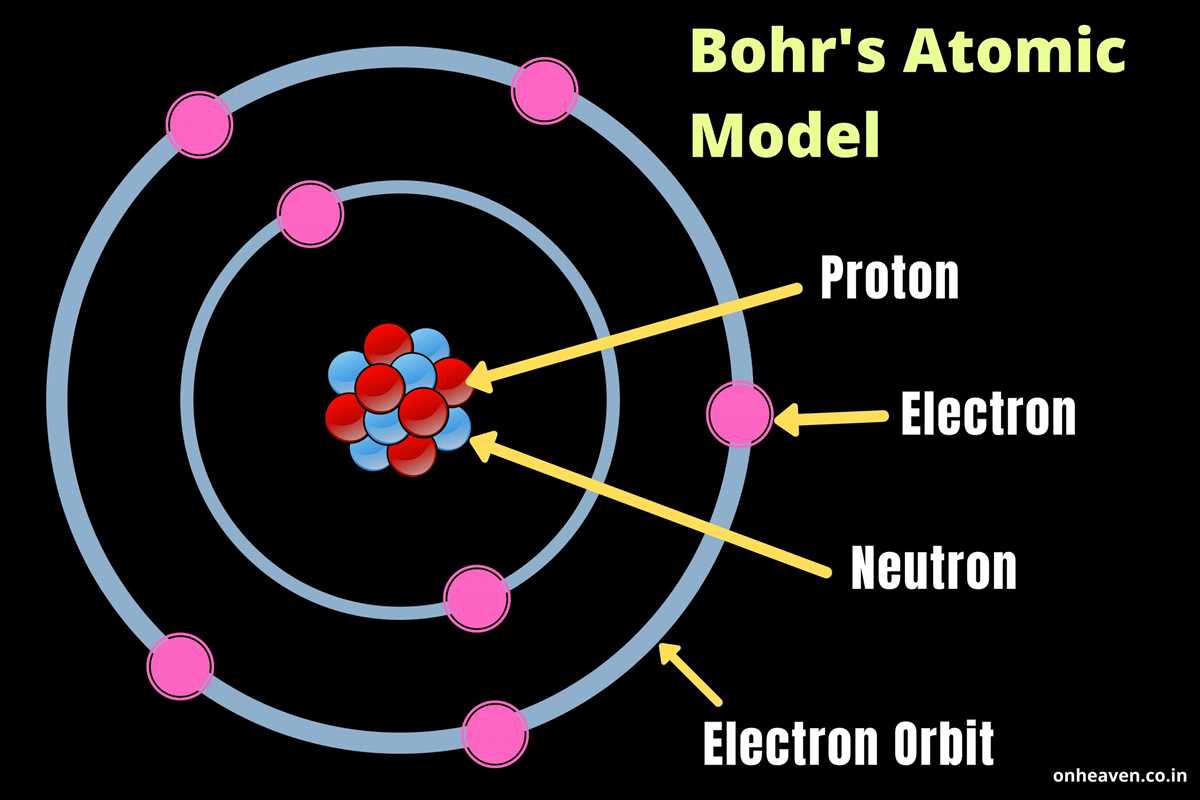
The arrangement of electrons in atoms plays a crucial role in determining the chemical properties and stability of an atom. In the periodic table, noble gases, also known as Group 18 elements, have a unique electron configuration that contributes to their exceptional stability.
Noble gases have a completely filled outermost electron shell, consisting of 8 valence electrons, except for helium, which has 2 valence electrons. This electron arrangement is referred to as noble gas configuration. The noble gas configuration is highly stable because it satisfies the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a complete octet in their outermost shell.
The stability of noble gases can be attributed to their filled electron shells. Because noble gases have a complete electron configuration, they have minimal reactivity and rarely form chemical bonds with other elements. This stability is a result of the balance between attractive forces within the atom and repulsive forces between electrons. The filled electron shells of noble gases create a strong electrostatic repulsion between the electrons, making it difficult for other atoms to interact and form bonds.
The noble gas configuration is often used as a reference point when discussing the electron arrangement of other elements. Elements that are located in the same period as a noble gas often have similar electron configurations, with the addition of one or more electrons. This similarity in electron configuration contributes to the similar chemical properties observed in elements within the same group.
In conclusion, noble gas configuration and the stability associated with it play a significant role in determining the chemical behavior of atoms. The completely filled outermost electron shell of noble gases contributes to their minimal reactivity and makes them highly stable. Understanding noble gas configuration helps to explain the periodic trends and similarities observed among elements in the periodic table.
Practice Problems and Answer Key
Here are some practice problems to test your understanding of the arrangement of electrons in atoms. Use the answer key provided to check your answers. Good luck!
- Problem 1: How many valence electrons does an atom of oxygen have?
- Problem 2: Write the electron configuration for an atom of nitrogen.
- Problem 3: How many electrons can occupy the 3d sublevel?
- Problem 4: What is the electron configuration for the element with atomic number 20?
- Problem 5: How many unpaired electrons are in an atom of sulfur?
Answer Key:
- Oxygen has 6 valence electrons.
- The electron configuration for nitrogen is 1s^2 2s^2 2p^3.
- The 3d sublevel can hold a maximum of 10 electrons.
- The electron configuration for the element with atomic number 20 (calcium) is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2.
- An atom of sulfur has 2 unpaired electrons.
These practice problems should help reinforce your understanding of the arrangement of electrons in atoms. Remember to refer to the periodic table and follow the rules for filling electron orbitals. Keep practicing and good luck with your studies!