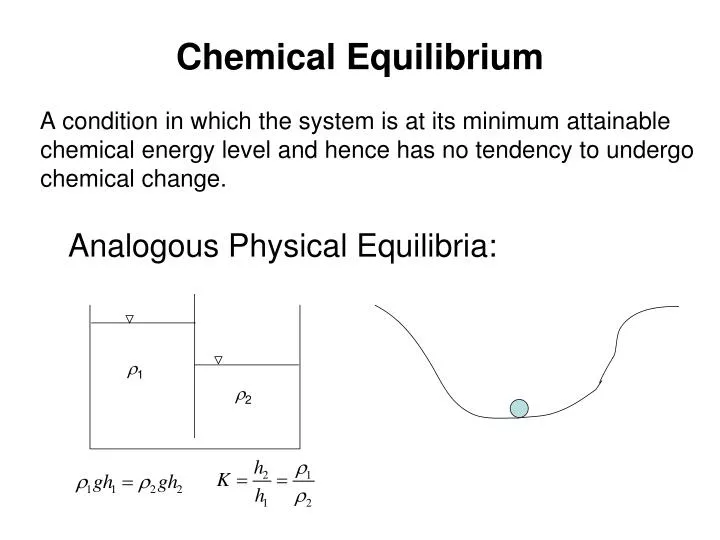
In chemical equilibrium, the rates of the forward and reverse reactions are equal, resulting in a state of balance. This concept is crucial in understanding how chemical reactions behave and can be applied to various real-world scenarios. In order to fully grasp the concept of chemical equilibrium, it is essential to practice solving equilibrium problems and analyzing the results.
Chemical equilibrium practice problems with answers in PDF format are a valuable resource for students and professionals alike. These practice problems provide an opportunity to test one’s understanding of equilibrium concepts and to reinforce the application of mathematical formulas and calculations. The answers provided in the PDF can serve as a reference to validate one’s solutions and identify any misconceptions or errors.
By practicing chemical equilibrium problems, individuals can enhance their problem-solving skills and gain confidence in their ability to tackle complex equilibrium scenarios. These practice problems cover a range of equilibrium concepts, including calculating equilibrium constants, determining equilibrium concentrations, and predicting the direction of the reaction based on changes in conditions. The step-by-step solutions provided in the PDF facilitate the learning process and help individuals develop a deeper understanding of the underlying principles of chemical equilibrium.
Chemical Equilibrium Practice Problems with Answers PDF
Chemical equilibrium is a crucial concept in the field of chemistry. It refers to a state in which the concentrations of reactants and products in a chemical reaction remain constant over time. Understanding chemical equilibrium is important because it allows scientists to predict the outcome of a reaction and design processes that maximize product yield.
To master the concept of chemical equilibrium, it is essential to practice solving equilibrium problems. One effective way to do this is by using practice problems with answers in PDF format. These PDFs provide a collection of equilibrium problems, ranging from simple to complex, along with step-by-step solutions.
By working through these practice problems, students can enhance their understanding of equilibrium concepts, such as the equilibrium constant, Le Chatelier’s principle, and the reaction quotient. The detailed solutions provided in the PDFs enable students to learn from their mistakes and grasp the underlying principles governing chemical equilibrium.
- For example, a typical problem in the PDF may involve calculating the equilibrium constant for a given reaction. The solution would outline the steps to determine the equilibrium concentrations of reactants and products, and then use these values to calculate the equilibrium constant.
- Another problem might involve predicting the effect of changing the temperature on the equilibrium position. The solution would explain how to apply Le Chatelier’s principle to determine whether the equilibrium shifts towards the reactants or the products.
Having access to practice problems with answers in PDF format allows students to practice equilibrium calculations at their own pace and review the solutions to gain a deeper understanding of the topic. It also serves as a valuable resource for educators who want to provide their students with additional practice materials.
In conclusion, chemical equilibrium practice problems with answers in PDF format are an invaluable tool for students and educators alike. They provide a structured way to practice equilibrium calculations and reinforce the fundamental concepts of chemical equilibrium.
Understanding Chemical Equilibrium
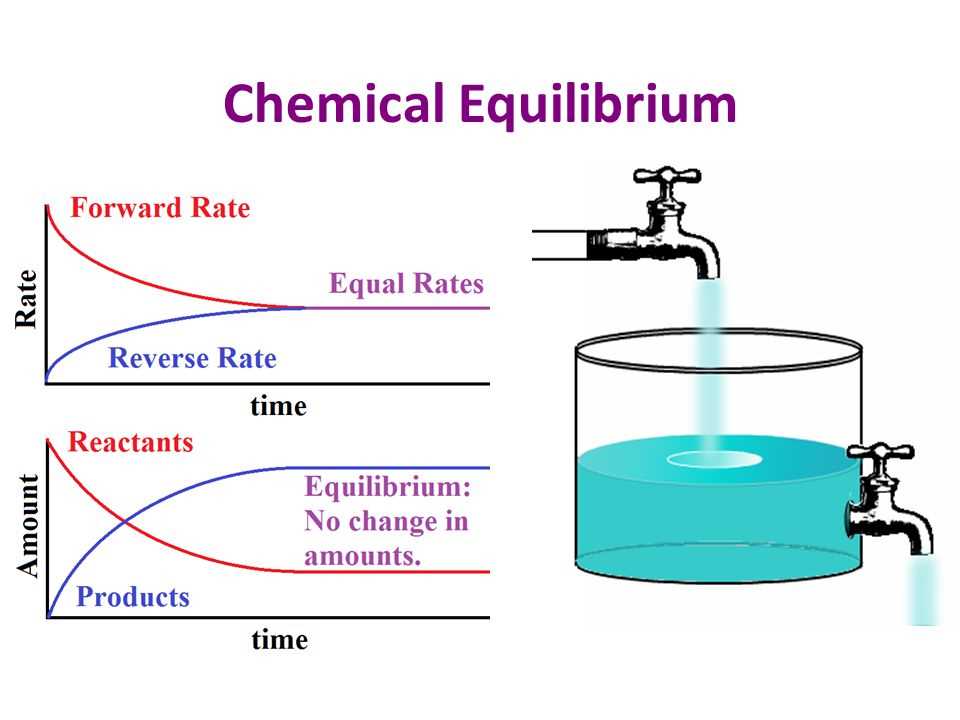
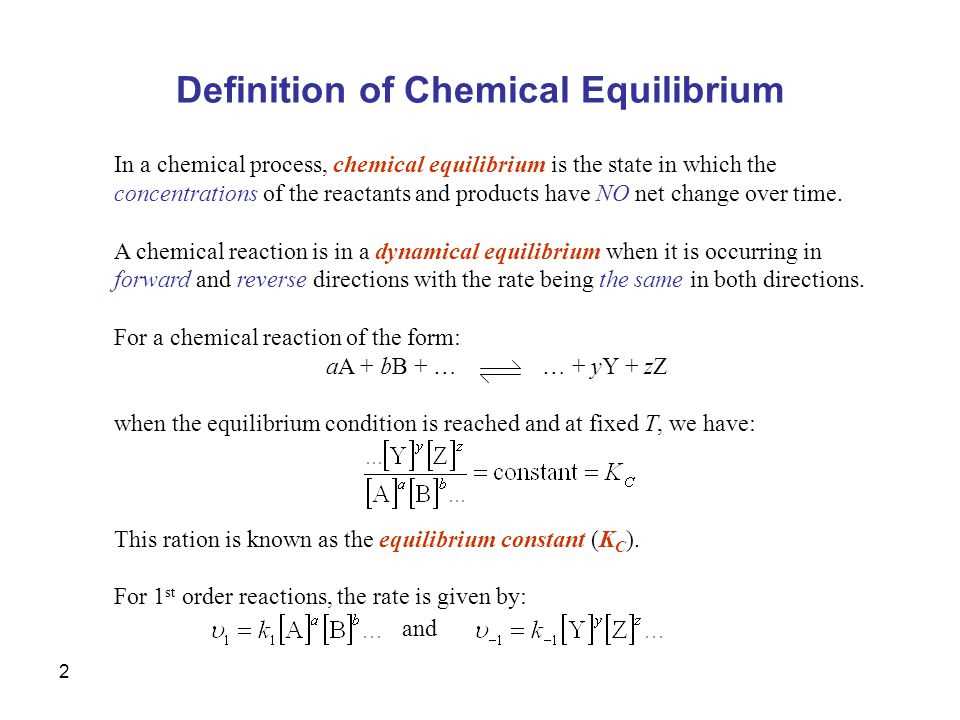
Chemical equilibrium is a fundamental concept in chemistry that describes the balance between the forward and backward reactions in a chemical system. It occurs when the rates of the forward and backward reactions are equal, resulting in no net change in the concentrations of the reactants and products over time. This state of balance is represented by the equilibrium constant (K), which is the ratio of the concentrations of the products to the concentrations of the reactants at equilibrium.
The principle of Le Chatelier’s can be applied to understand how various factors can affect the position of the equilibrium. According to Le Chatelier’s principle, adding or removing reactants or products, changing the temperature, or altering the pressure can shift the equilibrium position. For example, if more reactants are added to the system, the equilibrium will shift towards the product side to consume the excess reactants. Similarly, increasing the temperature will favor the endothermic reaction, while decreasing the temperature will favor the exothermic reaction.
To solve chemical equilibrium problems, it is essential to understand the concept of stoichiometry, which involves balancing chemical equations and calculating the relative amounts of reactants and products. This helps determine the equilibrium concentrations and allows for the calculation of equilibrium constants. The use of chemical equilibrium expressions and algebraic manipulations is crucial for solving these equilibrium problems.
In summary, understanding chemical equilibrium is essential in predicting how a chemical system will behave. Through the application of Le Chatelier’s principle, stoichiometry, and equilibrium expressions, we can determine the equilibrium concentrations and understand the factors that influence the equilibrium position. This knowledge is vital for various areas of chemistry, including reaction kinetics, thermodynamics, and industrial processes.
Calculating Equilibrium Constants
Understanding the concept of chemical equilibrium is essential in determining the composition of a system at equilibrium. One way to quantitatively describe equilibrium is by calculating the equilibrium constant, denoted as K. The equilibrium constant is a ratio of the concentrations (or partial pressures for gases) of the products to the concentrations (or partial pressures) of the reactants, with each concentration raised to the power of their respective stoichiometric coefficients.
To calculate the equilibrium constant, one must first write the balanced chemical equation for the reaction. Next, the concentrations (or partial pressures) of the reactants and products at equilibrium are determined. These values are then substituted into the equilibrium constant expression, and the equilibrium constant is calculated.
For example, consider the following reaction:
N2(g) + 3H2(g) ⇌ 2NH3(g)
To calculate the equilibrium constant K, we need to determine the concentrations of N2, H2, and NH3 at equilibrium. Let’s assume that at equilibrium, the concentrations are [N2] = 0.50 M, [H2] = 0.20 M, and [NH3] = 0.80 M. Substituting these values into the equilibrium constant expression, we have:
K = [NH3]^2 / ([N2][H2]^3) = (0.80)^2 / ((0.50)(0.20)^3) = 6.4
The equilibrium constant for this reaction is therefore 6.4. This indicates that at equilibrium, there are more products (NH3) than reactants (N2 and H2), suggesting that the reaction favors the formation of NH3.
Calculating equilibrium constants allows us to quantitatively analyze chemical reactions at equilibrium and provides valuable information about the relative concentrations of reactants and products. It also helps predict how changing conditions, such as temperature or pressure, would affect the equilibrium position.
Le Chatelier’s Principle and Equilibrium Shifts
Le Chatelier’s Principle is a fundamental principle in chemistry that helps us understand how chemical equilibrium shifts in response to changes in temperature, pressure, and concentration. According to this principle, when a system in equilibrium is subjected to an external stress, it will respond by shifting the equilibrium position to counteract or minimize the effect of the stress.
One of the most common ways to apply Le Chatelier’s Principle is by changing the concentration of reactants or products. If the concentration of a reactant is increased, the system will shift towards the product side to consume the excess reactant. Similarly, if the concentration of a product is increased, the system will shift towards the reactant side to produce more reactant and restore the equilibrium.
- When the concentration of a species is decreased, the system will shift in the direction that produces more of that species to replenish the decrease.
- When the concentration of a species is increased, the system will shift in the direction that consumes more of that species to decrease its concentration back to equilibrium.
Changes in temperature also have a significant impact on equilibrium shifts. For exothermic reactions, which release heat, increasing the temperature will shift the equilibrium towards the reactant side to absorb the excess heat. On the other hand, for endothermic reactions, which absorb heat, increasing the temperature will shift the equilibrium towards the product side to produce more heat and restore the equilibrium.
Pressure changes can also influence equilibrium shifts, but this primarily applies to gas-phase reactions. According to Le Chatelier’s Principle, if the pressure is increased, the system will shift towards the side with fewer moles of gas to decrease the overall pressure. Conversely, if the pressure is decreased, the system will shift towards the side with more moles of gas to increase the overall pressure.
In summary, Le Chatelier’s Principle provides a framework for understanding the behavior of chemical equilibrium by predicting how the system will adjust its composition in response to changes in temperature, pressure, and concentration. By applying this principle, chemists can manipulate and control chemical reactions to achieve desired outcomes.
Calculating Equilibrium Concentrations
In chemical equilibrium problems, it is often necessary to calculate the concentrations of various species at equilibrium. This can be done using the principles of the equilibrium constant and the stoichiometry of the reaction.
To calculate equilibrium concentrations, we must first write the balanced chemical equation for the reaction. Then, we can determine the equilibrium constant, which is denoted by K. The equilibrium constant relates the concentrations of the reactants and products at equilibrium.
Next, we need to determine the initial concentrations of the reactants and products. These initial concentrations can be given in the problem or calculated using the given information. In some cases, we may need to use an ICE table to calculate the initial concentrations.
After determining the initial concentrations, we can set up an expression for the equilibrium constant, K. This expression is written using the concentrations of the species at equilibrium. The equilibrium concentrations can be represented by the variables x, y, and z, depending on the stoichiometry of the reaction.
Once the expression for the equilibrium constant is set up, we can solve for the unknown equilibrium concentration(s). This can be done algebraically by rearranging the equation and solving for x, y, or z. In some cases, it may be necessary to use quadratic equations or approximations to find the solution.
Finally, we can substitute the calculated equilibrium concentration(s) back into the balanced chemical equation to verify that the reaction is at equilibrium. If the reaction is not at equilibrium, we may need to adjust the concentrations and repeat the calculations until the equilibrium conditions are met.
By following these steps, we can successfully calculate the equilibrium concentrations of species in a chemical reaction. This allows us to analyze and understand the behavior of chemical systems at equilibrium.
Factors Affecting Chemical Equilibrium
Chemical equilibrium is a dynamic state where the forward and reverse reactions occur at the same rate, resulting in no net change in the concentrations of reactants and products. Several factors can affect the position of equilibrium and alter the concentrations of reactants and products.
1. Concentration:
The concentration of reactant and product species can impact the equilibrium position. According to Le Chatelier’s principle, an increase in the concentration of a reactant or a decrease in the concentration of a product will shift the equilibrium towards the forward reaction to restore equilibrium. Conversely, an increase in the concentration of a product or a decrease in the concentration of a reactant will shift the equilibrium towards the reverse reaction.
2. Temperature:
Temperature is another significant factor that affects chemical equilibrium. Increasing the temperature of an exothermic reaction will shift the equilibrium towards the reactant side to absorb the excess heat. Similarly, decreasing the temperature of an endothermic reaction will shift the equilibrium towards the product side to generate more heat. However, these effects may vary depending on the specific reaction and its enthalpy change.
3. Pressure:
In gaseous systems, changes in pressure can also impact the equilibrium. An increase in pressure will shift the equilibrium towards the side with fewer gas molecules to decrease the pressure. Conversely, a decrease in pressure will shift the equilibrium towards the side with more gas molecules to increase the pressure. This relationship is based on the ideal gas law and the stoichiometry of the balanced equation.
4. Catalysts:
Catalysts do not directly affect the position of equilibrium but can increase the rate at which equilibrium is achieved. They provide an alternative pathway with lower activation energy, allowing the reactions to reach equilibrium faster. As catalysts facilitate both the forward and reverse reactions equally, they do not alter the equilibrium composition but enable the equilibrium to be reached more quickly.
5. Solvent:
The choice of solvent can influence the equilibrium by affecting the solubility and interaction of reactants and products. Different solvents can have different levels of polarity or acidity/basicity, which can impact the concentration and activity of species in solution. This, in turn, can affect the equilibrium position and the rate of the forward and reverse reactions.
In conclusion, the factors affecting chemical equilibrium include concentration, temperature, pressure, catalysts, and solvent choice. Understanding how these factors influence the equilibrium position can help in predicting and manipulating chemical reactions to achieve desired outcomes.
Practical Examples of Chemical Equilibrium
Chemical equilibrium is a fundamental concept in chemistry that is applicable to a wide range of real-life situations. Here are some practical examples that illustrate the concept of chemical equilibrium:
- Haber process: The synthesis of ammonia gas from nitrogen and hydrogen gases is a prime example of chemical equilibrium. The reaction involves the forward and reverse reactions, with both reactants and products present in the system. The equilibrium condition is reached when the rate of the forward reaction equals the rate of the reverse reaction.
- Acid-base reactions: Acid-base reactions, such as the dissociation of water into hydrogen and hydroxide ions, also exhibit chemical equilibrium. The reaction can proceed in both the forward and reverse directions, with the concentration of reactants and products determining the equilibrium position.
- Catalytic reactions: In catalytic reactions, a catalyst is used to speed up the rate of the reaction while remaining unchanged at the end of the reaction. The presence of a catalyst can shift the equilibrium position by providing an alternative reaction pathway with lower activation energy.
- Le Chatelier’s principle: This principle states that when a system at equilibrium is subjected to a change in temperature, pressure, or concentration, it will respond by shifting the equilibrium position to counteract the change. For example, if the concentration of a reactant is increased, the system will shift towards the product side to relieve the increased concentration.
| Chemical Equilibrium Example | Equilibrium Reaction |
|---|---|
| Haber Process | N2(g) + 3H2(g) ⇌ 2NH3(g) |
| Water Dissociation | H2O(l) ⇌ H+(aq) + OH-(aq) |
| Catalytic Reaction | 2NO(g) + O2(g) ⇌ 2NO2(g) |
In summary, chemical equilibrium is a fundamental concept that is observed in various chemical reactions. Understanding the concept of equilibrium is essential for predicting and explaining the behavior of chemical systems. By studying the practical examples of chemical equilibrium, we can better comprehend the dynamic nature of chemical reactions and their equilibrium positions.
Q&A:
What is a practical example of chemical equilibrium?
A practical example of chemical equilibrium is the reaction between nitrogen and hydrogen to form ammonia. In this reaction, nitrogen and hydrogen react to form ammonia, but ammonia can also decompose back into nitrogen and hydrogen. When the rate of the forward reaction is equal to the rate of the backward reaction, a state of equilibrium is reached.
What is another practical example of chemical equilibrium?
Another practical example of chemical equilibrium is the dissolution of carbon dioxide in water to form carbonic acid. The reaction between carbon dioxide and water can go both ways, with carbon dioxide dissolving in water to form carbonic acid, and carbonic acid decomposing into carbon dioxide and water. At equilibrium, the rate of dissolution of carbon dioxide is equal to the rate of decomposition of carbonic acid.
Can you give an example of a reversible reaction in chemical equilibrium?
A reversible reaction in chemical equilibrium is the reaction between acetic acid and ethanol to form ethyl acetate and water. In this reaction, acetic acid and ethanol can react to form ethyl acetate and water, but ethyl acetate and water can also react to form acetic acid and ethanol. At equilibrium, the rate of the forward reaction is equal to the rate of the backward reaction.
What is a practical example of a heterogeneous equilibrium?
A practical example of a heterogeneous equilibrium is the reaction between calcium carbonate and hydrochloric acid to form calcium chloride, carbon dioxide, and water. In this reaction, calcium carbonate reacts with hydrochloric acid to form calcium chloride, carbon dioxide, and water. However, the reaction can also go in the reverse direction, with calcium chloride, carbon dioxide, and water reacting to form calcium carbonate and hydrochloric acid. At equilibrium, there will be solid calcium carbonate in the reaction mixture, as it does not fully dissolve.
Can you provide an example of chemical equilibrium in the human body?
An example of chemical equilibrium in the human body is the transport of oxygen by hemoglobin in red blood cells. Hemoglobin can bind to oxygen molecules to form oxyhemoglobin, but it can also release oxygen molecules to tissues when needed. The balance between the binding and releasing of oxygen by hemoglobin is a dynamic equilibrium, ensuring that oxygen is delivered to tissues when required and released when needed.