
The Chemistry 1010 final exam is a comprehensive test that evaluates students’ understanding of the fundamental concepts and principles in chemistry. This exam serves as the culmination of a semester’s worth of learning and is designed to assess students’ knowledge and problem-solving skills in the subject.
Throughout the semester, students have been introduced to various topics, including atomic structure, chemical bonding, stoichiometry, gas laws, thermodynamics, and much more. The final exam covers all these areas and requires students to demonstrate their ability to apply the concepts they have learned in real-life situations and problem-solving scenarios.
The Chemistry 1010 final exam consists of multiple-choice questions, short answer questions, and in some cases, practical lab experiments. It is a timed exam that typically spans several hours. This test allows students to showcase their understanding of the subject matter and to apply the theories and concepts they have learned to solve problems and analyze data.
Chemistry 1010 Final Exam
The Chemistry 1010 final exam is a comprehensive assessment that tests students’ understanding of key concepts and principles covered throughout the course. It serves as an evaluation tool to measure students’ ability to apply their knowledge and skills in chemistry.
The final exam typically covers a wide range of topics, including atomic structure, chemical bonding, stoichiometry, gases, solutions, acids and bases, chemical reactions, thermodynamics, and kinetics. It requires students to demonstrate their understanding of the fundamental principles and theories in these areas and apply them to solving various types of problems.
The exam consists of multiple-choice questions, short-answer questions, and problem-solving questions. Students are expected to analyze and interpret data, perform calculations, and explain their reasoning in a clear and concise manner. It is important for students to review all the course material, including lecture notes, textbooks, and practice problems, in order to be well-prepared for the exam.
It is recommended that students allocate sufficient time for studying and review several weeks in advance of the exam. This will allow them to cover all the necessary material and identify any areas of weakness that require additional attention. It is also beneficial for students to actively engage in studying, such as through group discussions, practice exams, and seeking help from instructors or tutoring services.
In conclusion, the Chemistry 1010 final exam is a significant assessment that requires students to demonstrate their knowledge and understanding of various chemistry topics. By effectively preparing for the exam and utilizing appropriate study strategies, students can maximize their chances of success and achieve a satisfactory grade in the course.
Chemistry Basics
Chemistry is the scientific study of matter and the changes it undergoes. It is a fundamental branch of science that helps us understand the world around us at the molecular level. To understand chemistry, we need to have a basic understanding of its key concepts and principles.
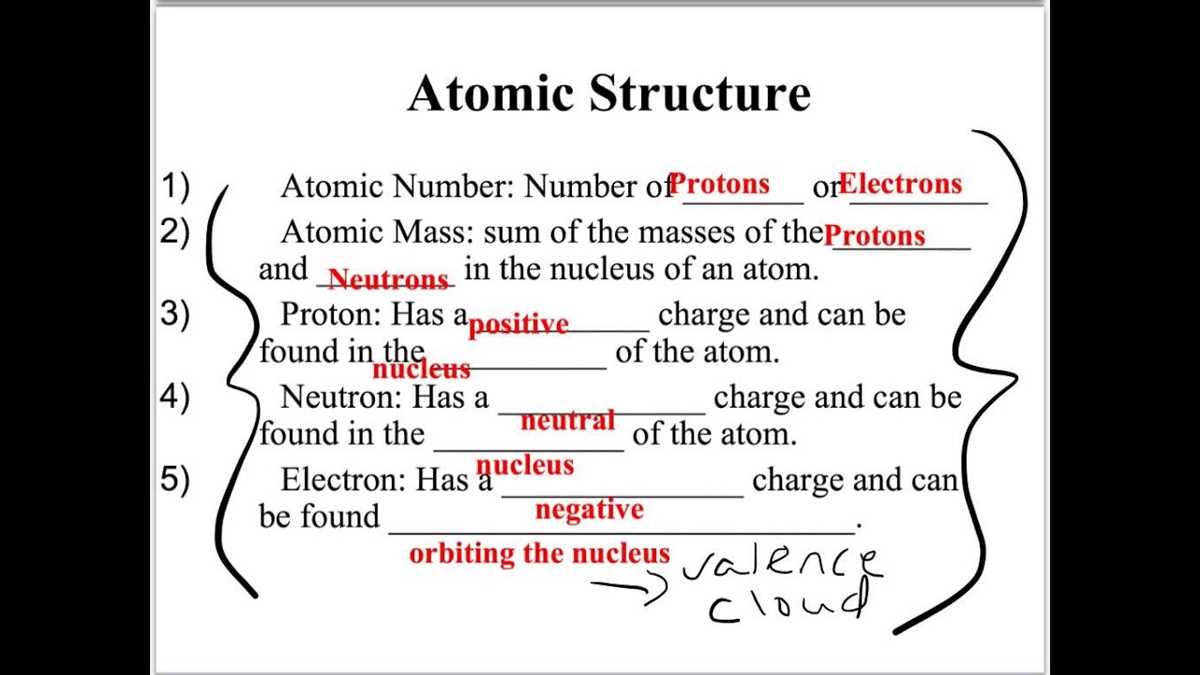
One of the fundamental concepts in chemistry is the atom. Atoms are the building blocks of matter and are composed of protons, neutrons, and electrons. Protons have a positive charge, neutrons are neutral, and electrons have a negative charge. The number of protons determines the element’s identity, while the number of neutrons and electrons can vary.
In chemistry, we use the periodic table to organize the elements. The periodic table provides valuable information about each element, such as its atomic number, atomic mass, and chemical symbol. It is divided into periods (rows) and groups (columns), with elements in the same group having similar chemical properties.
Chemical reactions are another important concept in chemistry. A chemical reaction occurs when substances undergo a change to form new substances with different properties. In a chemical reaction, the reactants are the starting substances, and the products are the substances formed as a result of the reaction. Chemical reactions can be represented by chemical equations, which show the relationship between the reactants and products.
Understanding the basics of chemistry is crucial for further exploration in the field. It allows us to comprehend the composition, properties, and behavior of matter, as well as the changes it undergoes. Whether it is studying the structure of molecules, analyzing the behavior of gases, or investigating the properties of acids and bases, a strong foundation in chemistry is essential for any aspiring scientist or student.
Atomic Structure and Periodic Table
The study of atomic structure is fundamental to understanding the properties and behavior of elements. Atoms are the building blocks of matter and consist of a nucleus, which contains protons and neutrons, surrounded by electrons orbiting in specific energy levels. The number of protons in the nucleus determines the element. For example, carbon atoms have six protons, while oxygen atoms have eight.
The periodic table is a visual representation of all known elements arranged in order of increasing atomic number. It provides a comprehensive overview of the periodic trends and patterns in chemical properties. Elements in the periodic table are organized into periods (horizontal rows) and groups (vertical columns). The rows correspond to the number of electron shells in an atom, while the columns represent the number of valence electrons.
Periodic trends
- Atomic radius decreases from left to right across a period and increases down a group.
- Ionization energy increases from left to right across a period and decreases down a group.
- Electronegativity follows a similar pattern to ionization energy.
- Metallic character decreases from left to right across a period and increases down a group.
Valence electrons
The number of valence electrons determines an atom’s chemical behavior and its ability to form bonds with other atoms. Valence electrons are the electrons in the outermost energy level of an atom. Elements in the same group of the periodic table have similar properties because they have the same number of valence electrons. For example, group 1 elements (alkali metals) all have one valence electron, making them highly reactive.
Summary
Understanding atomic structure and the periodic table is crucial for studying and predicting the behavior of elements and compounds. The periodic table provides a systematic way to organize and categorize elements based on their properties, while atomic structure explains why elements exhibit certain behaviors. By applying the principles of atomic structure and periodic trends, scientists can better understand and manipulate chemical reactions and create new materials with specific properties.
Chemical Bonding
Chemical bonding refers to the process by which atoms are joined together to form molecules or compounds. This bond is formed through the sharing, gaining, or losing of electrons. There are three main types of chemical bonds: covalent bonds, ionic bonds, and metallic bonds.
A covalent bond is formed when two atoms share electrons in order to achieve a stable electron configuration. This type of bond is typically found between nonmetals and is characterized by the sharing of electron pairs. Covalent bonds can be either polar or nonpolar, depending on the electronegativity difference between the atoms involved.
An ionic bond is formed when one or more electrons are transferred from one atom to another. This results in the formation of positively charged cations and negatively charged anions. Ionic bonds are typically found between metals and nonmetals and are characterized by the attraction between oppositely charged ions.
Metallic bonding occurs when metal atoms share their valence electrons in a “sea” of delocalized electrons. This allows the atoms to move freely within the structure, giving metals their unique properties such as conductivity and malleability.
These different types of chemical bonds play a crucial role in determining the properties and behavior of substances. Understanding chemical bonding is essential in explaining the behavior of molecules and compounds, as well as in predicting their chemical reactions.
Chemical Reactions
Chemical reactions are fundamental processes that occur in all living and non-living systems. These reactions involve the rearrangement of atoms to create new substances with different properties. Understanding chemical reactions is crucial in the study of chemistry, as it allows scientists to predict and control the behavior of substances.
In a chemical reaction, reactants are transformed into products through the breaking and forming of chemical bonds. This process can be represented by a chemical equation, which shows the reactants on the left side and the products on the right side. The coefficients in the equation represent the stoichiometric ratios, indicating the number of molecules or atoms involved in the reaction.
Chemical reactions can be classified into different types, including synthesis, decomposition, combustion, and displacement reactions. Synthesis reactions involve the combination of two or more substances to form a new compound. Decomposition reactions, on the other hand, involve the breakdown of a compound into smaller substances. Combustion reactions occur when a substance reacts with oxygen to produce heat and light. Displacement reactions involve the exchange of atoms or ions between two compounds.
Chemical reactions are governed by several principles and laws, such as the law of conservation of mass and the law of definite proportions. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, only rearranged. The law of definite proportions states that a chemical compound always contains the same elements in the same ratio by mass.
In conclusion, chemical reactions play a crucial role in understanding the behavior and transformation of substances. By studying and manipulating these reactions, scientists can develop new materials, understand biological processes, and improve industrial processes.
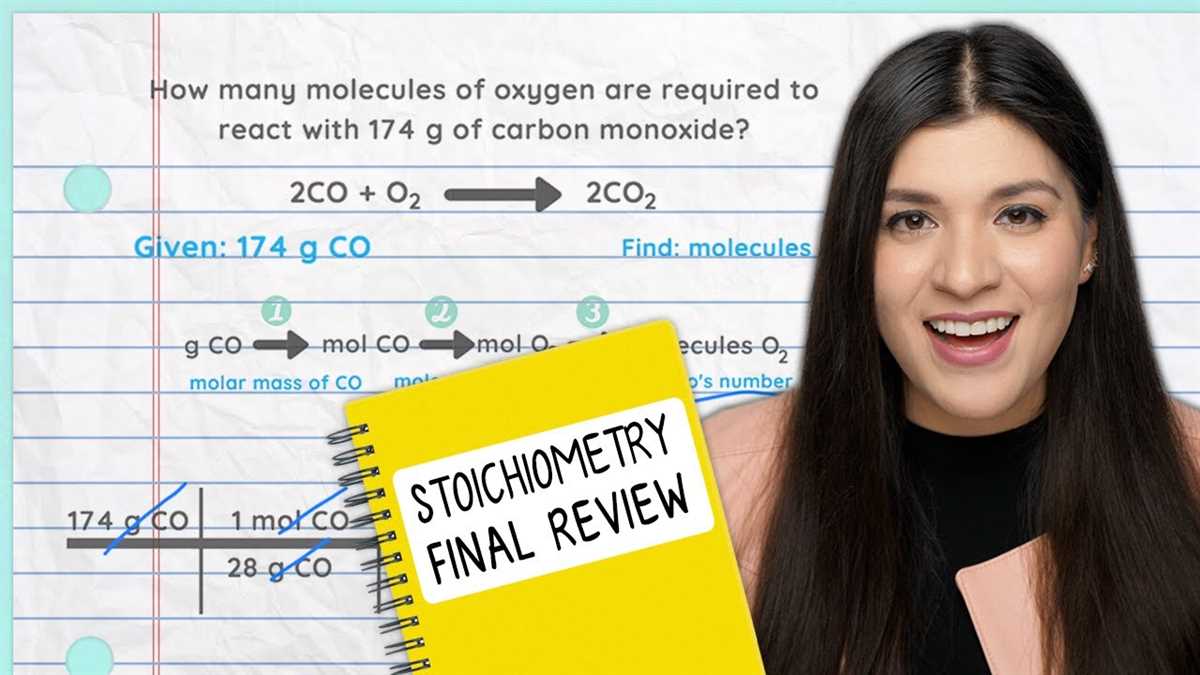
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the quantitative relationship between reactants and products in chemical reactions. It is essential for understanding and predicting the amounts of substances involved in a chemical reaction, as well as determining the limiting reactant and the theoretical yield of a reaction. Stoichiometry is based on the principle of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
In stoichiometry, balanced chemical equations are used to relate the number of moles, mass, and volume of substances involved in a reaction. The coefficients in the balanced equation represent the mole ratio between reactants and products. This allows chemists to calculate the amount of one substance in a reaction based on the known amount of another substance.
Stoichiometry calculations can involve various units, including moles, grams, liters, and molecules. These calculations are typically done using dimensional analysis, which involves converting between different units using conversion factors derived from the coefficients in the balanced equation.
- Limited reactant: Stoichiometry also helps determine the limiting reactant in a chemical reaction, which is the reactant that is completely consumed and limits the amount of product that can be formed.
- Theoretical yield: Theoretical yield is the maximum amount of product that can be obtained in a reaction based on stoichiometric calculations.
- Excess reactant: Stoichiometry can also be used to determine the amount of excess reactant present after a reaction is complete.
Overall, stoichiometry is an essential tool in chemistry for understanding the quantitative aspects of chemical reactions and calculating the amounts of substances involved.
Conclusion
Acids, bases, and pH are fundamental concepts in chemistry that play a vital role in our everyday lives. Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases are substances that release hydroxide ions (OH-) when dissolved in water. The pH scale is used to measure the acidity or alkalinity of a solution, with values ranging from 0 to 14.
Understanding acids, bases, and pH is important in various fields, including medicine, environmental science, and industrial processes. In medicine, pH monitoring helps diagnose conditions such as acid reflux and urinary tract infections. In environmental science, knowledge of pH levels in water bodies is crucial for assessing water quality and its impact on aquatic life. In industrial processes, controlling pH is essential for optimizing chemical reactions and product quality.
It is important to note that acids and bases can react with each other to neutralize the pH of a solution. This process is known as a neutralization reaction, where an acid reacts with a base to form water and a salt. The strength of an acid or base is determined by its ability to donate or accept protons, which is measured by the dissociation constant.
Overall, a deep understanding of acids, bases, and pH is essential for anyone studying chemistry or entering fields where these concepts are applicable. It allows us to comprehend the behavior of various substances, analyze chemical reactions, and make informed decisions in scientific and everyday situations.