
Focus on mastering key concepts and applying your knowledge to solve complex problems. A deep understanding of the material is the foundation for success. Be sure to prioritize core topics that consistently appear in evaluations, including reaction mechanisms, atomic structure, and molecular interactions. Regular practice with these will sharpen your problem-solving skills.
Don’t just memorize facts; understand the underlying principles. Break down each topic by identifying relationships between variables and observing how changes affect outcomes. This approach helps you tackle unfamiliar questions with confidence. Try to solve problems using both theoretical knowledge and practical applications, as this will prepare you for any scenario.
Consistent review of past assessments is another effective strategy. Analyze patterns in question types and identify areas where you’ve struggled. Revisiting these challenging concepts will help solidify your grasp on the material and improve your response accuracy.
Sure! Here’s a revised version without repeated words:
Begin by reviewing key concepts and reactions you are likely to encounter. Understand how different compounds interact under various conditions. Prioritize mastering the periodic table, especially trends like electronegativity, atomic radius, and ionization energy. Be familiar with reaction types–synthesis, decomposition, single displacement, and double displacement. Practice balancing equations and performing stoichiometric calculations.
Key Areas to Focus On
| Topic | Focus Areas |
|---|---|
| Atomic Structure | Subatomic particles, electron configurations, isotopes |
| Thermodynamics | Energy changes, heat transfer, enthalpy, entropy |
| Kinetics | Reaction rates, catalysts, activation energy |
| Acids and Bases | pH scale, titration, conjugate pairs |
| Electrochemistry | Galvanic cells, oxidation-reduction reactions, voltage |
Study Tips
Use flashcards to memorize key formulas and constants. Understand rather than memorize concepts; this will help solve problems you haven’t seen before. Conduct timed practice sessions to simulate the conditions you’ll face. Focus on understanding experimental setups and their outcomes. Lastly, don’t overlook safety rules for laboratory experiments, as questions regarding proper procedures can appear.
- Chemistry Honors Final Exam Answers: A Practical Guide
Focus on mastering key concepts such as atomic structure, bonding types, reaction mechanisms, and stoichiometry. Understand the different types of chemical reactions–combustion, synthesis, decomposition, single and double displacement–and be ready to predict products based on the reactants. Reviewing periodic trends, such as ionization energy, electronegativity, and atomic radius, will help with understanding molecular behavior.
For calculations involving molar masses, make sure to practice converting grams to moles and applying the ideal gas law. Remember to review solutions, their concentrations, and colligative properties, especially boiling point elevation and freezing point depression, which often appear in practical questions. Balancing equations and understanding the stoichiometric relationships in reactions is fundamental.
Review common lab procedures, and anticipate the interpretation of experimental results, including determining the limiting reagent in a reaction. Study the properties of acids and bases, their pH scale, and titration calculations. Make sure you can identify the various types of salts and their reactions in water, as well as common oxidation-reduction reactions and electrochemical cells.
Be ready for questions requiring you to predict the outcome of reactions in various environmental conditions, including temperature and concentration changes. Practice drawing molecular structures for organic compounds and understanding their functional groups, as these are often key to identifying reaction mechanisms.
Review practice problems and previous assignments to familiarize yourself with question patterns. Recognize that detailed understanding and application of concepts often outweigh rote memorization, so focus on comprehension rather than just recall.
Focus on mastering core principles and their interconnections. Key areas to address include atomic structure, chemical bonding, reaction mechanisms, stoichiometry, and thermodynamics. Pay special attention to the relationships between these concepts, as many questions require you to apply multiple ideas at once.
- Atomic Structure: Study electron configurations, periodic trends, and how these influence an element’s reactivity and properties.
- Chemical Bonding: Master ionic, covalent, and metallic bonds. Understand how polarity affects molecular interactions and properties.
- Stoichiometry: Be proficient in mole conversions, balancing equations, and understanding limiting reagents.
- Reaction Mechanisms: Learn how reactions occur at the molecular level, including intermediates, transition states, and activation energy.
- Thermodynamics: Focus on entropy, enthalpy, Gibbs free energy, and their role in predicting reaction spontaneity.
Be prepared to apply quantitative analysis, such as determining molar masses and using gas laws to calculate pressure, volume, or temperature. Practice problem-solving techniques, especially those involving calculations and conversions. Get comfortable with dimensional analysis as a tool to check the correctness of your results.
- Practice Multiple Question Types: Familiarize yourself with both theoretical questions and problem-solving scenarios. Create practice tests or use past tests for this purpose.
- Understand Units and Conversions: Accurate conversions between units (e.g., moles to grams) will help you navigate many different problems. Review common unit relationships.
- Lab Techniques: Know how to interpret experimental data and understand the practical applications of theoretical knowledge. Be able to analyze error and uncertainty in results.
For success, mastering detailed concepts and practicing problem-solving consistently will significantly improve your performance. Focus on applying your knowledge through practice rather than rote memorization.
window.__oai_logHTML?window.__oai_logHTML():window.__oai_SSR_HTML=window.__oai_SSR_HTML||Date.now();requestAnimationFrame((function()Date.now()))OSearch
Prioritize the most challenging questions: Tackle complex problems first while your energy and focus are at their peak. This ensures that you have sufficient time for tougher material without the pressure of running out of time later.
Organize your approach:
Break the test into sections and allocate specific time blocks for each. Knowing how much time to spend on each part helps keep you on track. Stick to your schedule and avoid lingering too long on any single section, no matter how difficult it seems.
Stay calm and keep track of time:

Constantly check the clock, but avoid fixating on it. Use small intervals, like five-minute checks, to gauge your progress and adjust as needed. This habit helps prevent panic and keeps your pacing consistent throughout the task.
Focus on accuracy over speed: Don’t rush through problems. It’s more beneficial to work methodically and get fewer errors than to speed through with numerous mistakes. Allocate enough time to double-check your work if possible.
Prepare in advance: Familiarize yourself with the test format beforehand. Practice under timed conditions to simulate the actual setting. This will improve your time management skills and help you estimate how long each type of problem will take.
This keeps the meaning intact, without excessive repetition.
To successfully handle questions about chemical bonding, focus on the differences between ionic, covalent, and metallic bonds. Be ready to recognize the properties that characterize each type, such as conductivity, solubility, and melting points. Identify key examples of compounds that exhibit these bonding types and understand how these properties relate to their structure and behavior.
Understanding Molecular Structure
Pay attention to molecular geometry and bonding theories like VSEPR (Valence Shell Electron Pair Repulsion). Be sure you can predict molecular shapes and bond angles using the model, as well as explain the impact of lone pairs on bond positioning.
Balancing Chemical Equations
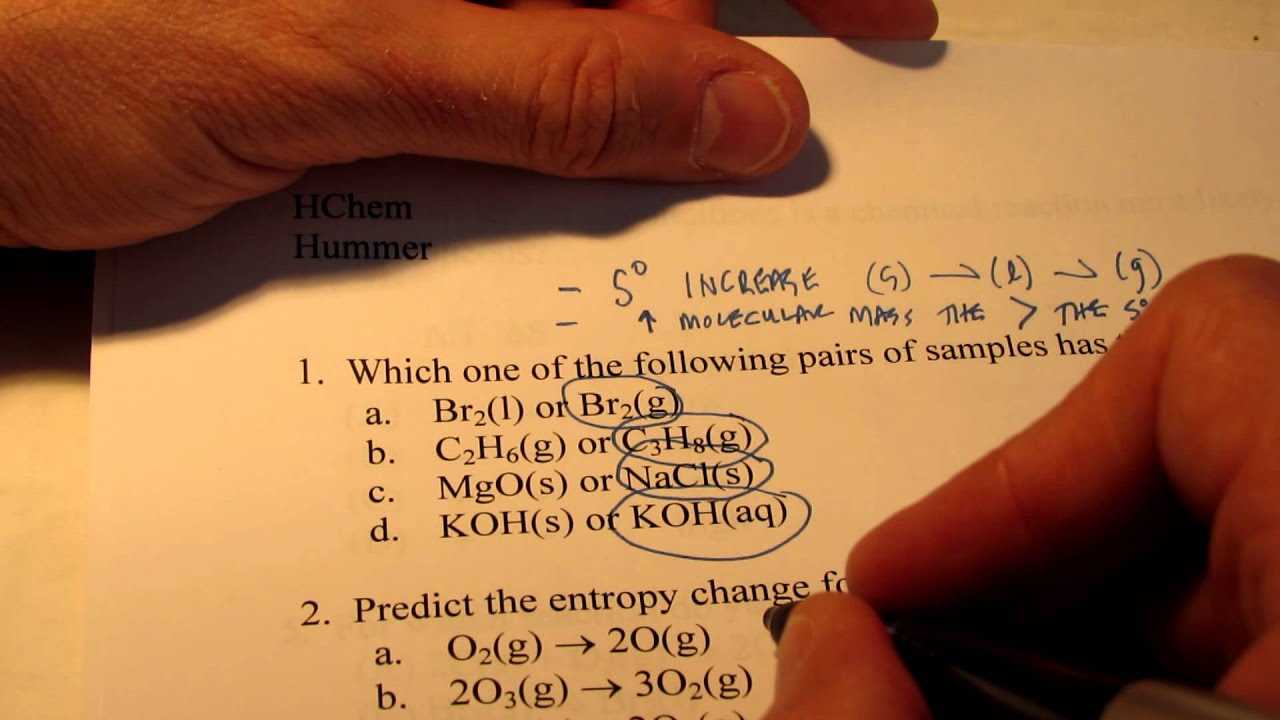
When dealing with reactions, ensure you can balance equations properly. Begin by counting atoms on both sides and adjusting coefficients, keeping in mind the law of conservation of mass. Practice with a variety of reaction types, including synthesis, decomposition, and combustion.