
Chemistry unit 4 test is an important assessment for students studying chemistry. This test typically covers topics related to the periodic table, chemical bonding, and chemical reactions.
During this test, students are expected to demonstrate their understanding of the different elements in the periodic table, their properties, and their organization. They will also be tested on their knowledge of chemical bonds, including the different types of bonds and the forces that hold atoms together.
Furthermore, the test will assess students’ understanding of chemical reactions, including concepts such as balancing chemical equations and identifying different types of reactions. It will also require students to apply their knowledge to solve problems and answer conceptual questions.
Preparing for the chemistry unit 4 test involves reviewing class notes, textbook materials, and practicing with sample questions. It is also important for students to understand the key concepts and to be able to apply them to real-world examples. By doing so, students will be well-prepared for the test and can approach it with confidence.
Overview of Chemistry Unit 4 Test

Welcome to the overview of the Chemistry Unit 4 test! In this unit, we will be focusing on several key topics that are fundamental to our understanding of chemistry. These topics include bonding, chemical reactions, and stoichiometry.
Bonding: One of the main concepts we will be exploring in this unit is chemical bonding. We will learn about different types of chemical bonds, such as ionic and covalent bonds, and how they form between atoms. Additionally, we will study the properties of compounds formed by these bonds, such as their melting points, boiling points, and solubilities.
Chemical Reactions: Another important area of focus is chemical reactions. We will extensively cover the different types of reactions, including synthesis, decomposition, single replacement, and double replacement reactions. Through these discussions, we will learn how to write and balance chemical equations, and how to predict the products of a given reaction.
Stoichiometry: Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. We will learn how to use stoichiometric calculations to determine the amount of reactants or products involved in a reaction, as well as perform calculations related to percent yield and limiting reactants.
Throughout this unit, we will apply our knowledge of these topics to solve various problems and complete hands-on activities. It is important to review your notes, complete practice problems, and seek clarification on any concepts you find challenging. By mastering the content in this unit, you will be well-prepared for the upcoming test. Good luck!
Examining the topics and goals
Chemistry unit 4 test is an important assessment that covers a range of topics and goals. This test is designed to evaluate students’ understanding of key concepts and principles in chemistry, as well as their ability to apply this knowledge to solve problems and analyze data.
One of the main topics covered in this unit is chemical reactions. Students will learn about the different types of chemical reactions, such as synthesis, decomposition, combustion, and precipitation. They will also explore the concept of stoichiometry, which involves balancing chemical equations and determining the quantities of reactants and products involved in a reaction. Understanding these topics is essential for students to be able to predict the outcomes of chemical reactions and calculate reaction yields and measurements.
Another important topic in this unit is the behavior of gases. Students will study the basic principles of gas laws, including Boyle’s law, Charles’ law, and the combined gas law. They will also learn about the ideal gas law and its application in solving problems related to gas pressure, volume, and temperature. Additionally, students will explore the concept of gas stoichiometry, which involves calculating the quantities of reactants and products involved in a gas-phase reaction. This topic is crucial for students to understand the behavior of gases and make predictions about their properties and interactions.
The overall goal of this unit is to provide students with a solid foundation in the fundamental principles of chemistry. By examining the topics of chemical reactions and the behavior of gases, students will develop a deep understanding of core concepts and gain the necessary skills to analyze and solve complex problems in chemistry. This unit also aims to enhance students’ critical thinking and analytical skills, as they will be required to apply their knowledge to real-world scenarios and experimental data. Ultimately, the chemistry unit 4 test is designed to assess students’ mastery of these topics and their ability to think critically and apply their knowledge in the field of chemistry.
Understanding Chemical Reactions
Chemical reactions are fundamental processes that occur in nature and play a crucial role in our everyday lives. They involve the rearrangement of atoms to form new substances with different properties. To fully understand chemical reactions, it is important to grasp the concept of atoms and how they interact with each other.
At the heart of every chemical reaction are the reactants and products. Reactants are the substances that undergo a change, while products are the new substances formed as a result of the reaction. The reactants and products are represented using chemical formulas, which consist of symbols representing the different elements involved and subscripts indicating the number of atoms present.
Chemical reactions can be classified into several categories, including synthesis reactions, decomposition reactions, single displacement reactions, double displacement reactions, and combustion reactions. Each type of reaction follows a specific pattern and involves different types of reactants and products.
- Synthesis reactions occur when two or more substances combine to form a single, more complex substance. These reactions are often represented by the general equation: A + B → AB.
- Decomposition reactions involve the breakdown of a single substance into two or more simpler substances. The general equation for decomposition reactions is: AB → A + B.
- Single displacement reactions occur when an element replaces another element in a compound. The general equation for single displacement reactions is: A + BC → AC + B.
- Double displacement reactions involve the exchange of ions between two compounds. The general equation for double displacement reactions is: AB + CD → AD + CB.
- Combustion reactions involve the rapid combination of a substance with oxygen, usually resulting in the release of heat and light. These reactions are often represented by the general equation: fuel + oxygen → carbon dioxide + water.
Understanding chemical reactions is essential for a wide range of disciplines, including chemistry, biology, and environmental science. It allows us to predict the outcome of reactions, design new materials, and develop strategies for waste management and pollution control. By studying the principles of chemical reactions, scientists can uncover new insights into the natural world and make significant advancements in various fields.
Exploring Different Types and Characteristics
In chemistry, there are many different types of substances and compounds that exhibit unique characteristics. These substances can be classified into various categories based on their composition and properties. Some of the commonly studied types include elements, compounds, mixtures, and solutions.
Elements are pure substances that cannot be broken down into simpler substances by ordinary chemical means. They consist of a single type of atom and have specific physical and chemical properties. There are currently 118 known elements, each with its own unique set of characteristics.
Compounds, on the other hand, are substances that are made up of two or more different elements chemically combined in a fixed ratio. Unlike elements, compounds have different properties from their constituent elements and can only be separated through chemical reactions. Examples of common compounds include water (H2O) and sodium chloride (NaCl).
Mixtures are combinations of two or more substances that are physically mixed together, but not chemically bonded. The components of a mixture can be separated by physical means, such as filtration or distillation. Mixtures can be classified as homogeneous (uniform composition throughout) or heterogeneous (non-uniform composition). Examples of mixtures include air (a mixture of gases) and saltwater (a mixture of salt and water).
Solutions are a special type of homogeneous mixture where one substance (the solute) is dissolved in another substance (the solvent). The solute particles are evenly distributed and cannot be easily separated by filtration or distillation. Some common examples of solutions include sugar dissolved in water and salt dissolved in vinegar.
In conclusion, exploring the different types and characteristics of substances in chemistry helps us understand their behavior and interactions. By studying elements, compounds, mixtures, and solutions, scientists can gain insights into the properties and reactions of matter, leading to advancements in various fields of science and technology.
Chemical Equations and Stoichiometry

In chemistry, chemical equations are used to represent the reaction between different substances. They provide a concise and systematic way to describe the starting materials, the products formed, and the stoichiometry of the reaction. A chemical equation consists of reactants on the left-hand side, separated by plus signs, and products on the right-hand side, also separated by plus signs.
Understanding stoichiometry is crucial when working with chemical equations. Stoichiometry is the quantitative relationship between the amounts of reactants and products in a chemical reaction. It allows chemists to determine the amount of each substance needed or produced in a reaction, as well as the ratios in which they are combined.
In a balanced chemical equation, the number of atoms of each element must be the same on both sides. This is achieved by adjusting the coefficients in front of each molecule. Stoichiometry calculations involve using the balanced equation to determine the mole ratios between different substances, which can then be used to calculate the mass, volume, or number of particles involved in the reaction.
Stoichiometry is widely used in various areas of chemistry, including determining the yield of a reaction, predicting the amount of product that can be obtained, and calculating the concentration of solutions. It plays a crucial role in understanding and predicting chemical reactions, as well as in industrial processes and laboratory experiments.
To summarize, chemical equations and stoichiometry are fundamental concepts in chemistry. They allow us to represent and understand the transformations of substances during chemical reactions, as well as calculate the amounts of reactants and products involved. Understanding these concepts is essential for any student or practitioner of chemistry.
Learning how to balance and solve equations
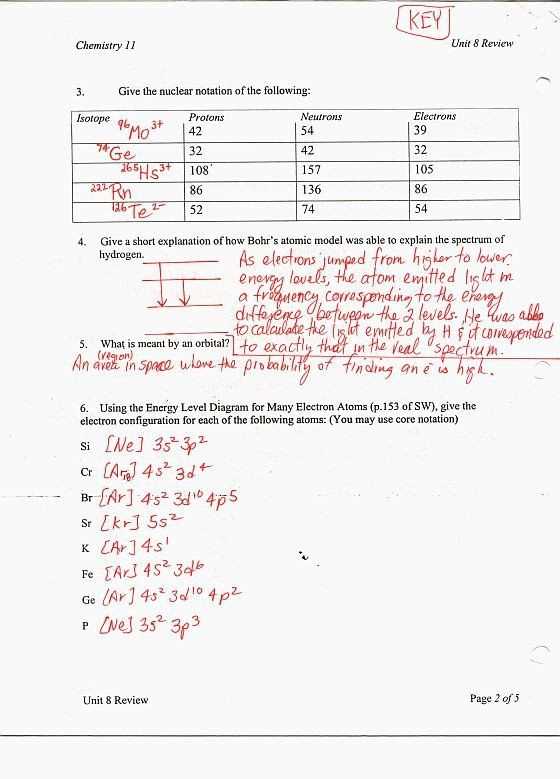
One of the fundamental skills in chemistry is the ability to balance and solve equations. Chemical equations represent the conversion of reactants into products during a chemical reaction. These equations provide a visual representation of what is happening at the molecular level.
When balancing equations, the goal is to ensure that the number of atoms of each element is the same on both sides of the equation. This is achieved by adjusting the coefficients in front of each compound or element. Balancing equations requires a thorough understanding of chemical formulas, the periodic table, and the concept of conservation of mass.
To balance an equation, follow these steps:
- Write down the unbalanced equation, including the chemical formulas of all the reactants and products.
- Identify the elements present in the equation and count the number of atoms of each element on both sides.
- Start balancing the equation by adjusting the coefficients in front of the compounds or elements. Begin with the elements that appear in the fewest number of compounds.
- Continue adjusting the coefficients until the number of atoms of each element is the same on both sides of the equation.
- Double-check your balanced equation to ensure that all the elements are balanced and the law of conservation of mass is satisfied.
Once an equation is balanced, it can be used to solve various problems in chemistry, such as calculating the amount of reactants or products needed or produced during a reaction. Balancing and solving equations is an essential skill that lays the foundation for understanding more complex chemical concepts and calculations.
Energy Changes in Chemical Reactions

In chemistry, energy changes play a fundamental role in chemical reactions. During a chemical reaction, bonds between atoms are broken and new bonds are formed, resulting in the formation of different substances. These bond-breaking and bond-forming processes involve the transfer or release of energy, which can be observed as changes in temperature, light, or sound.
Endothermic reactions are chemical reactions that absorb heat from the surroundings. In these reactions, the reactants have a lower energy level than the products, and energy is transferred from the surroundings to the reactants to break the existing bonds. As a result, the surroundings become cooler. An example of an endothermic reaction is the reaction between ammonium nitrate and water, which is commonly used in cold packs to provide a cooling effect.
Exothermic reactions, on the other hand, release heat to the surroundings. In exothermic reactions, the reactants have a higher energy level than the products, and energy is released as new bonds are formed. This energy release can be observed as an increase in temperature in the surroundings. Combustion reactions, such as the burning of gasoline or wood, are examples of exothermic reactions.
Understanding and studying energy changes in chemical reactions is important for various reasons. It allows scientists to predict whether a reaction is likely to occur spontaneously or require an input of energy. It also helps in designing and optimizing chemical processes, such as in the production of fuels or pharmaceuticals. Overall, energy changes in chemical reactions provide valuable insights into the underlying mechanisms and dynamics of chemical transformations.
Investigating Endothermic and Exothermic Reactions
During the Chemistry unit 4 test, one of the topics explored was endothermic and exothermic reactions. These reactions involve the absorption or release of energy, respectively. In this section, we will summarize the key points discussed and the results obtained from investigating these reactions.
Endothermic Reactions:
- Endothermic reactions are those in which energy is absorbed from the surroundings.
- One example of an endothermic reaction is the decomposition of ammonium chloride.
- During the investigation, it was observed that the temperature of the reactants decreased as the reaction took place.
- The decrease in temperature indicates that energy was absorbed from the surroundings to drive the reaction.
Exothermic Reactions:
- Exothermic reactions are those in which energy is released to the surroundings.
- One example of an exothermic reaction is the combustion of methane.
- When investigating exothermic reactions, it was found that the temperature of the reactants increased as the reaction occurred.
- The increase in temperature indicates that energy was released as a product of the reaction.
In conclusion, investigating endothermic and exothermic reactions allowed us to understand how energy is involved in chemical reactions. Endothermic reactions absorb energy, resulting in a decrease in temperature, while exothermic reactions release energy, leading to an increase in temperature. These observations provide valuable insights into the thermodynamics of chemical reactions and can be applied in various fields, such as energy production and environmental studies.