
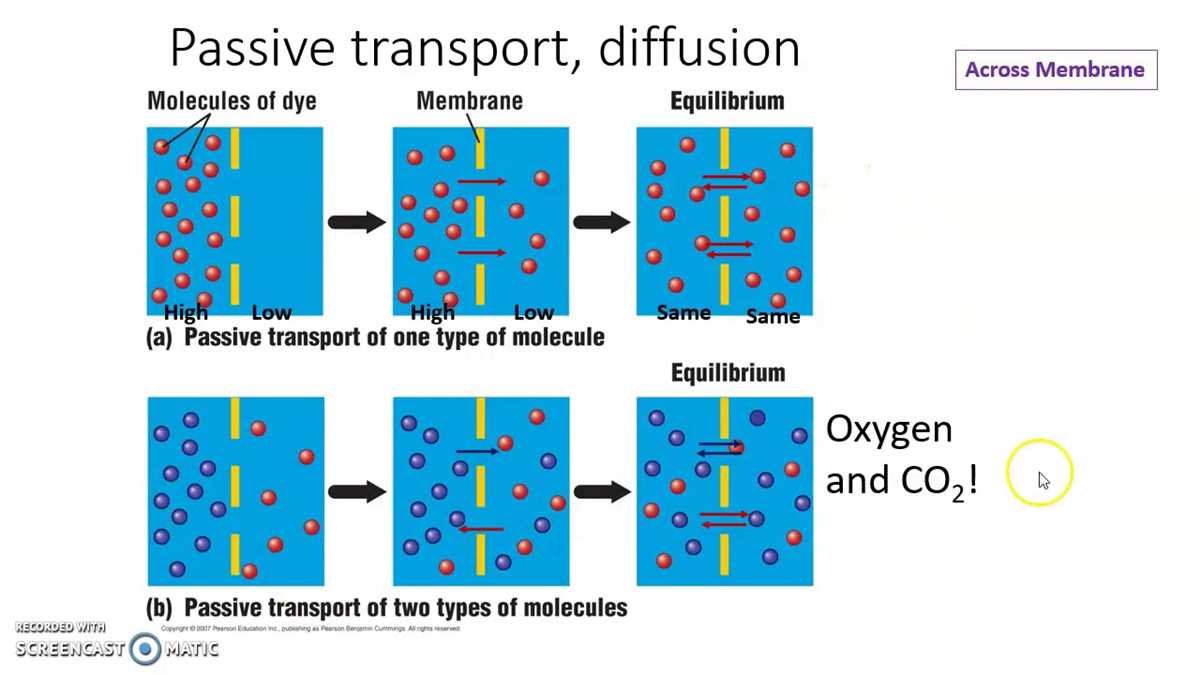
Diffusion is the process by which molecules move from an area of higher concentration to an area of lower concentration. One of the ways diffusion occurs in living organisms is through a semi-permeable membrane. In this lab, we will explore the process of diffusion through a membrane and analyze the results.
During the first part of the lab, we observed the diffusion of iodine molecules through a dialysis membrane. The dialysis membrane is a semi-permeable membrane that allows small molecules to pass through while blocking larger molecules. We placed a solution of iodine in a beaker and immersed the dialysis membrane in it. Over time, we observed the iodine molecules diffusing through the membrane and into the surrounding water. This diffusion was evident by the color change in the water as the iodine molecules spread out.
In part 2 of the lab, we focused on the factors that affect the rate of diffusion through a membrane. We conducted several experiments where we varied the concentration of the solute on one side of the membrane, the temperature, and the size of the molecules. By measuring the rate of diffusion using various indicators, such as the rate of color change or the distance traveled by the molecules, we were able to draw conclusions about the factors that influence diffusion through a membrane.
Experimental setup and procedure
The following is a description of the experimental setup and procedure used in the diffusion through a membrane lab.
Materials
- Glass beaker
- Membrane (such as a dialysis tubing)
- Starch solution
- Iodine solution
- Water
- Clip or rubber band
- Pipette
Setup
First, a glass beaker is filled with water and placed on a level surface. A piece of membrane (such as a dialysis tubing) is soaked in water to ensure it is hydrated and stretched out. The membrane is then securely attached to the beaker using a clip or rubber band, creating a barrier between the two compartments of the beaker.
Procedure
The starch solution is prepared by dissolving a measured amount of starch powder in water. The iodine solution is also prepared by diluting an appropriate amount of iodine in water. Once the solutions are prepared, a small amount of starch solution is placed inside the membrane using a pipette. The beaker is then covered to prevent evaporation.
The diffusion process is observed and measured over a set period of time. During this time, the iodine solution is added to the beaker outside the membrane. As the iodine molecules are smaller than the starch molecules, they are able to pass through the membrane and react with the starch, resulting in a color change. The color change is indicative of how far and how quickly the iodine molecules diffuse through the membrane.
The diffusion process is typically repeated with different concentrations of starch and iodine solutions to investigate the effect of concentration on the rate of diffusion. Additionally, the temperature of the water in the beaker can be varied to study its impact on diffusion.
Overall, the experimental setup and procedure allow for the observation and quantification of diffusion through a membrane, providing valuable insights into the properties of different solutes and membranes.
Results and observations

During the diffusion through a membrane lab, several key observations and results were recorded. Firstly, it was observed that the diffusion rate was influenced by the concentration gradient. In the experiments where the concentration gradient was higher, the diffusion rate was faster. This indicates that the concentration gradient plays a crucial role in determining the rate of diffusion across a membrane.
Additionally, it was noted that the size of the molecules also affected the diffusion rate. Larger molecules took longer to diffuse across the membrane compared to smaller molecules. This suggests that the size of the molecules is an important factor in determining how easily they can pass through a membrane.
Furthermore, it was observed that the type of membrane used also influenced the diffusion rate. In experiments where a semi-permeable membrane was used, the diffusion rate was slower compared to experiments where a permeable membrane was used. This indicates that the permeability of the membrane can significantly impact the rate of diffusion.
Overall, these results demonstrate the complex nature of diffusion through a membrane and highlight the importance of factors such as concentration gradient, molecule size, and membrane permeability in determining the rate of diffusion. Further experimentation and analysis would be necessary to fully understand and explain these observations.
Analysis and Interpretation
During the diffusion through a membrane lab experiment, the rate of diffusion of molecules across a semi-permeable membrane was observed. The experiment consisted of two setups, one with a dialysis bag filled with a glucose and starch solution, and the other with a beaker containing iodine. The purpose of the experiment was to investigate the factors affecting the rate of diffusion.
Based on the observations, it can be concluded that the rate of diffusion is influenced by the concentration gradient and the size of the molecules. In the first setup, the color change of the iodine solution indicated the presence of glucose molecules diffusing out of the dialysis bag. This suggests that glucose, being a smaller molecule than starch, was able to pass through the pores of the membrane more easily. The rate of diffusion was also observed to be faster when the concentration of glucose in the bag was higher.
Furthermore, the rate of diffusion was found to be slower for starch molecules. This can be explained by the larger size of starch molecules, which makes it more difficult for them to pass through the membrane pores. As a result, the iodine solution only turned slightly purple, indicating a lower concentration of starch diffusing into the beaker.
The experiment showed that the rate of diffusion is directly proportional to the concentration gradient. This is consistent with the concept of diffusion, where molecules move from an area of higher concentration to an area of lower concentration. Additionally, the experiment highlighted the selective permeability of the dialysis bag membrane, allowing only certain molecules to pass through.
In conclusion, the analysis and interpretation of the diffusion through a membrane lab experiment demonstrated the influence of concentration gradient and molecular size on the rate of diffusion. These findings provide valuable insights into the mechanisms of diffusion and the role of membrane permeability. Further experiments and investigations can be conducted to explore other factors affecting diffusion and expand our understanding of this fundamental process.
Further Research and Implications
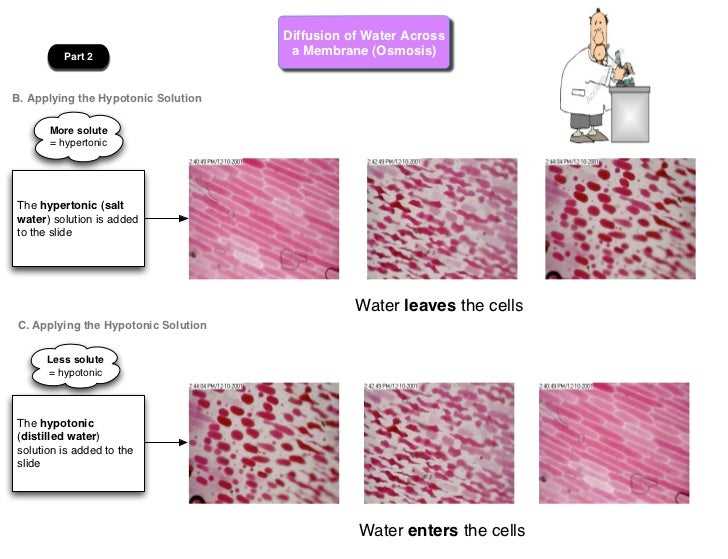
The diffusion through a membrane lab answers part 2 provided valuable insights into the process of diffusion and its relation to membrane permeability. However, there are still many avenues for further research in this field.
One possible area for future investigation is the effect of temperature on membrane permeability. It would be interesting to determine how changes in temperature affect the rate of diffusion, as well as the selectivity of the membrane. This research could have implications for various industries, such as pharmaceuticals and food preservation, where understanding the effect of temperature on diffusion is crucial.
Another area for further research is the impact of different solutes on membrane permeability. The diffusion through a membrane lab focused on the diffusion of potassium permanganate, but it would be valuable to study the diffusion of other solutes as well. By examining the diffusion of various solutes, we can gain a better understanding of the factors that influence membrane permeability and potentially develop new applications in fields such as drug delivery and water treatment.
In summary, the diffusion through a membrane lab answers part 2 provided important insights into the process of diffusion and its relation to membrane permeability. However, there is still much to be explored in this field, such as the effect of temperature on membrane permeability and the impact of different solutes. Further research in these areas has the potential to expand our understanding of diffusion and its applications in various industries.