
Understanding electron configuration is essential in chemistry as it helps us understand the behavior and properties of elements. Electron configuration refers to the arrangement of electrons in an atom’s different energy levels or orbitals. It follows a specific set of rules and principles, which can be challenging to grasp at first.
To help students practice and reinforce their understanding of electron configuration, worksheets are often assigned. These worksheets provide exercises for students to practice writing electron configurations for different elements based on their atomic number.
Having access to the answers for these practice worksheets is extremely helpful for students. It allows them to check their work, identify any mistakes, and learn from them. It also provides a reference for students to understand the correct electron configuration, ensuring they learn the principles accurately.
In this article, we will provide the answers to a popular electron configuration practice worksheet. By referring to these answers, students can assess their knowledge and improve their understanding of electron configuration. Let’s dive into the answers and explore the world of electron configuration together!
Understanding Electron Configurations: A Practice Worksheet
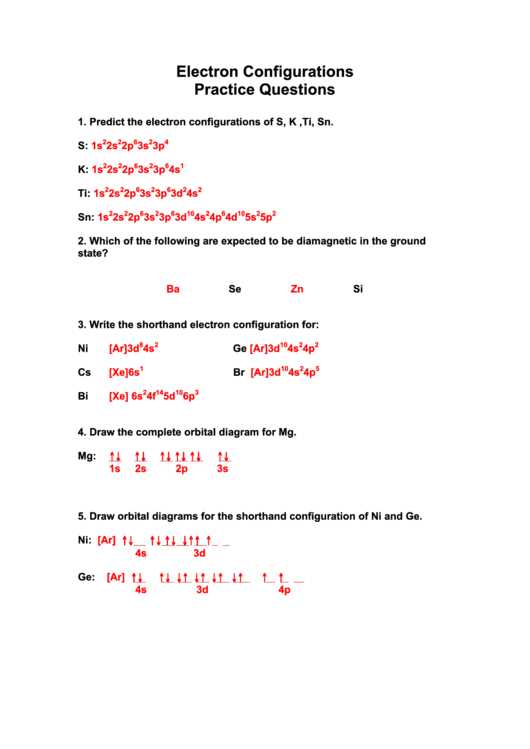
Electron configurations are an important concept in chemistry that help us understand how electrons are organized within an atom. By knowing the electron configuration of an atom, we can determine its stability, reactivity, and other properties. To practice and solidify our understanding of electron configurations, we can use a practice worksheet that provides various elements to work with.
The practice worksheet typically consists of a list of elements and their atomic numbers. We start by writing the atomic number of each element, which indicates the number of protons and electrons in the atom. To determine the electron configuration, we follow a set of rules that guide us in filling the electron shells and subshells. These rules include the Aufbau principle, Pauli exclusion principle, and Hund’s rule.
For example, let’s consider the element carbon (atomic number 6). We start by filling the lowest energy level, which is the first shell with two electrons. Next, we move to the second shell, which can hold up to eight electrons. Therefore, we place the remaining four electrons in the second shell, with two in the 2s subshell and two in the 2p subshell. The electron configuration of carbon is therefore 1s2 2s2 2p2.
By practicing with different elements and their electron configurations, we can familiarize ourselves with the patterns and trends that exist. We can observe how the electron configuration changes across different periods and groups of the periodic table. It is important to remember that electron configurations are not arbitrary, but rather follow specific rules and patterns based on the principles of quantum mechanics.
Sample Practice Worksheet:
- Element: Oxygen (O)
- Atomic Number: 8
- Electron Configuration: 1s2 2s2 2p4
- Element: Sodium (Na)
- Atomic Number: 11
- Electron Configuration: 1s2 2s2 2p6 3s1
Exploring the Basics of Electron Configurations

Understanding electron configurations is fundamental in the study of chemistry. An electron configuration is a description of how electrons are distributed among the energy levels and orbitals of an atom. It provides valuable information about the chemical behavior of an element and helps in predicting its properties.
Electron configurations are represented using a series of numbers and letters. The numbers represent the energy levels, and the letters represent the sublevels or orbitals within those energy levels. The electron configuration of an element can be determined by following a set of rules and principles.
One of the key principles is the aufbau principle, which states that electrons fill the lowest energy levels and sublevels first before moving to higher energy levels. This means that the electron configuration of an element is built up by adding electrons to each successive energy level and sublevel in a specific order.
Another important rule is the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. This means that each electron within a sublevel must have a unique combination of spin and orientation.
Understanding electron configurations allows chemists to predict the chemical reactivity, bonding behavior, and physical properties of elements. It provides a framework for understanding why certain elements are more stable or reactive than others. By analyzing electron configurations, chemists can also determine the number of valence electrons, which play a crucial role in determining the element’s chemical behavior.
In conclusion, electron configurations are a fundamental concept in chemistry. They provide valuable information about the distribution of electrons within an atom and help in predicting the properties and behavior of elements. By understanding electron configurations, chemists can unlock the secrets of the periodic table and make meaningful connections between different elements and their chemical reactivity.
Mastering the Aufbau Principle
The Aufbau principle is a fundamental concept in chemistry that explains how electrons are arranged in an atom. It is based on the idea that electrons fill up orbitals in ascending order of their energy levels, with the lowest energy levels being filled first.
Understanding the Aufbau Principle
The Aufbau principle is essential for understanding electron configurations, which describe how electrons occupy atomic orbitals. Each orbital can hold a maximum of two electrons with opposite spins. The Aufbau principle helps determine the order in which these orbitals are filled, based on their increasing energy levels.
- The lowest energy level, known as the 1s orbital, is filled first with two electrons.
- The next energy level, 2s, is filled with two electrons before moving to the 2p orbitals.
- The 2p orbitals are filled one at a time before moving to the next energy level, 3s.
Mastering Electron Configurations
By mastering the Aufbau principle, you can easily determine the electron configuration of an atom. This knowledge is crucial for understanding the chemical behavior of elements and their reactivity.
Electron configuration practice worksheets provide valuable exercises that allow you to apply the Aufbau principle to various elements. These worksheets often include instructions on how to fill up the orbitals step by step, following the Aufbau principle. By practicing these worksheets, you can develop a solid understanding of electron configurations and enhance your problem-solving skills in chemistry.
Conclusion
The Aufbau principle is a fundamental concept in chemistry, guiding the arrangement of electrons in an atom. By understanding and mastering this principle, you can confidently determine the electron configuration of any element. Practicing electron configuration worksheets will further enhance your understanding and problem-solving abilities in chemistry.
The Hund’s Rule: Putting Electrons in Their Places
When it comes to electron configuration, one important principle to understand is the Hund’s Rule. This rule dictates how electrons are placed in the available orbitals within an atom. It provides guidelines for filling up orbitals with electrons in a way that minimizes the overall energy of the system. By following the Hund’s Rule, scientists can accurately determine the electron configuration of an atom.
In simple terms, the Hund’s Rule states that when filling up orbitals of the same energy level, electrons will occupy different orbitals with the same spin before pairing up. This means that electrons will first fill each orbital with one spin before pairing up with an opposite spin. For example, if there are three available orbitals, the first electron will occupy one orbital with its spin up, the second electron will occupy a second orbital with its spin up, and the third electron will occupy the third orbital with its spin up as well.
This rule is derived from the principle that electrons repel each other due to their negative charge. By occupying different orbitals before pairing up, electrons can maximize their distance from each other, reducing the repulsion between them and ultimately lowering the overall energy of the system. This concept is known as the exchange energy. It is important to note that the Hund’s Rule only applies to orbitals of the same energy level, known as degenerate orbitals. For orbitals of different energy levels, electrons will first fill up the lower energy orbitals before moving on to the higher energy ones.
Overall, the Hund’s Rule plays a crucial role in understanding the electron configuration of atoms. By following this rule, scientists can accurately predict and describe the distribution of electrons within an atom’s orbitals, providing important insights into the chemical behavior and properties of elements.
Demystifying Orbital Notations

Understanding electron configurations and orbital notations is essential in the study of chemistry. These notations provide a way to represent the distribution of electrons within an atom’s energy levels and sublevels. By deciphering these notations, scientists can determine an element’s properties, behavior, and reactivity.
The most common way to represent electron configurations is through orbital notation, which uses arrows and lines to represent electrons in different orbitals. Each orbital can hold a maximum of two electrons, and they are represented by arrows facing up or down. Arrows pointing up represent electrons with a spin quantum number of +1/2, while arrows pointing down represent electrons with a spin quantum number of -1/2.
Orbital notations follow the Aufbau principle, which states that electrons fill the lowest energy orbitals first. This means that when writing the electron configuration for an element, you start with the lowest energy level (1s) and work your way up. The energy levels are represented by numbers, such as 1, 2, 3, etc., while the sublevels are represented by letters, such as s, p, d, and f.
For example, the electron configuration for carbon is 1s2 2s2 2p2. This means that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital. The superscripts represent the number of electrons in each orbital.
By understanding and practicing orbital notation, chemists can predict an element’s behavior and interactions with other elements. It is an essential tool for understanding the periodic table and the properties of various elements. So, demystify orbital notations and unlock the secrets of the electron configurations!
Practicing Electron Configurations with Examples
Understanding electron configurations is essential in chemistry as it helps us visualize and predict the behavior of elements. It provides information about how electrons are arranged in an atom and the energy levels they occupy. Practicing electron configurations is crucial in mastering this concept. Let’s explore some examples to further solidify our understanding.
Example 1: Carbon (C) has an atomic number of 6. To determine its electron configuration, we start by filling the lowest energy level, which is the 1s orbital. This energy level can hold a maximum of 2 electrons. Thus, the electron configuration of carbon can be written as 1s^2. Next, we move to the next energy level, which is the 2s orbital. Again, this energy level can hold a maximum of 2 electrons, so the electron configuration becomes 2s^2. Finally, we fill the remaining orbitals in the second energy level, which are the 2p orbitals. Each p orbital can hold a maximum of 2 electrons, resulting in the electron configuration of 2p^2. Hence, the complete electron configuration of carbon is 1s^2 2s^2 2p^2.
Example 2: Oxygen (O) has an atomic number of 8. Following the same process as in the previous example, we start by filling the 1s orbital, which can hold 2 electrons. The electron configuration becomes 1s^2. Moving to the second energy level, the 2s orbital can also hold 2 electrons, resulting in the electron configuration of 2s^2. We then fill the 2p orbitals, where each p orbital can hold 2 electrons. Thus, the electron configuration becomes 2p^4. The complete electron configuration of oxygen is 1s^2 2s^2 2p^4.
Practicing electron configurations with different elements allows us to become familiar with the patterns and rules governing their arrangement. It also helps us recognize trends and similarities between elements. By gaining proficiency in electron configurations, we can accurately predict an element’s reactivity, bonding behavior, and its position in the periodic table.
Summary:
- Electron configurations provide information about the arrangement of electrons in an atom.
- Practicing electron configurations is crucial to understand the behavior of elements.
- Examples of carbon and oxygen demonstrate the process of determining electron configurations.
- Practicing electron configurations helps recognize patterns and trends among elements.
- Proficiency in electron configurations aids in predicting reactivity, bonding behavior, and position in the periodic table.
Answers to the Electron Configuration Practice Worksheet
1. What is the electron configuration for carbon?
The electron configuration for carbon is 1s2 2s2 2p2.
2. What is the electron configuration for nitrogen?
The electron configuration for nitrogen is 1s2 2s2 2p3.
3. What is the electron configuration for oxygen?
The electron configuration for oxygen is 1s2 2s2 2p4.
4. What is the electron configuration for fluorine?
The electron configuration for fluorine is 1s2 2s2 2p5.
5. What is the electron configuration for phosphorus?
The electron configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3.
6. What is the electron configuration for sulfur?
The electron configuration for sulfur is 1s2 2s2 2p6 3s2 3p4.
7. What is the electron configuration for chlorine?
The electron configuration for chlorine is 1s2 2s2 2p6 3s2 3p5.
8. What is the electron configuration for argon?
The electron configuration for argon is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6.
It is important to remember that electron configurations follow certain rules and patterns. The electron configuration represents the arrangement of electrons in the energy levels and sublevels of an atom. By understanding electron configurations, we can better understand an atom’s chemical behavior and reactivity.