
When different substances are exposed to high temperatures, they emit light in unique patterns. This phenomenon can be used to identify the composition of unknown materials. Through a process that involves heating compounds, specific reactions occur, leading to the release of distinctive colors. These colors help in identifying the elements present in a substance. Understanding these reactions is a fundamental skill in many scientific experiments, offering a clear visual representation of chemical components.
Principles Behind the Color Emission

The reason behind the release of light when certain compounds are heated lies in the behavior of their electrons. When atoms are exposed to heat, their electrons jump to higher energy levels. As they return to their original state, energy is released in the form of light. This emitted light corresponds to specific wavelengths, producing distinct colors that are characteristic of certain substances.
Common Color Patterns
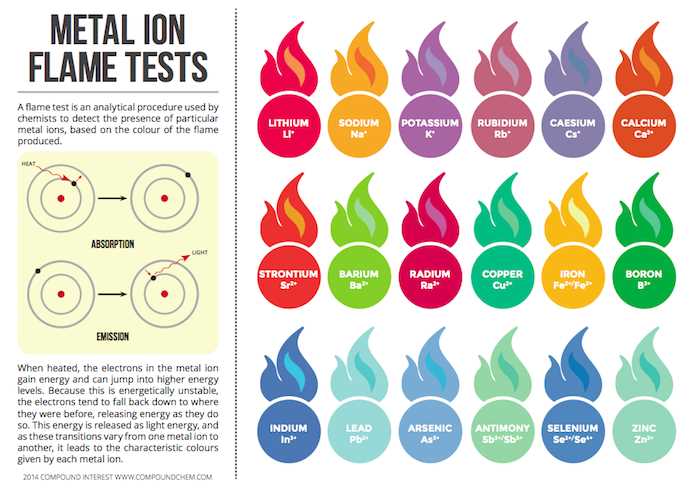
Each type of ion generates a unique emission spectrum when heated. Some common color reactions include:
- Red – Often produced by lithium and strontium ions.
- Blue – Associated with copper and cobalt ions.
- Green – Seen with compounds of barium and copper.
- Yellow – Common in sodium and potassium compounds.
Conducting the Procedure
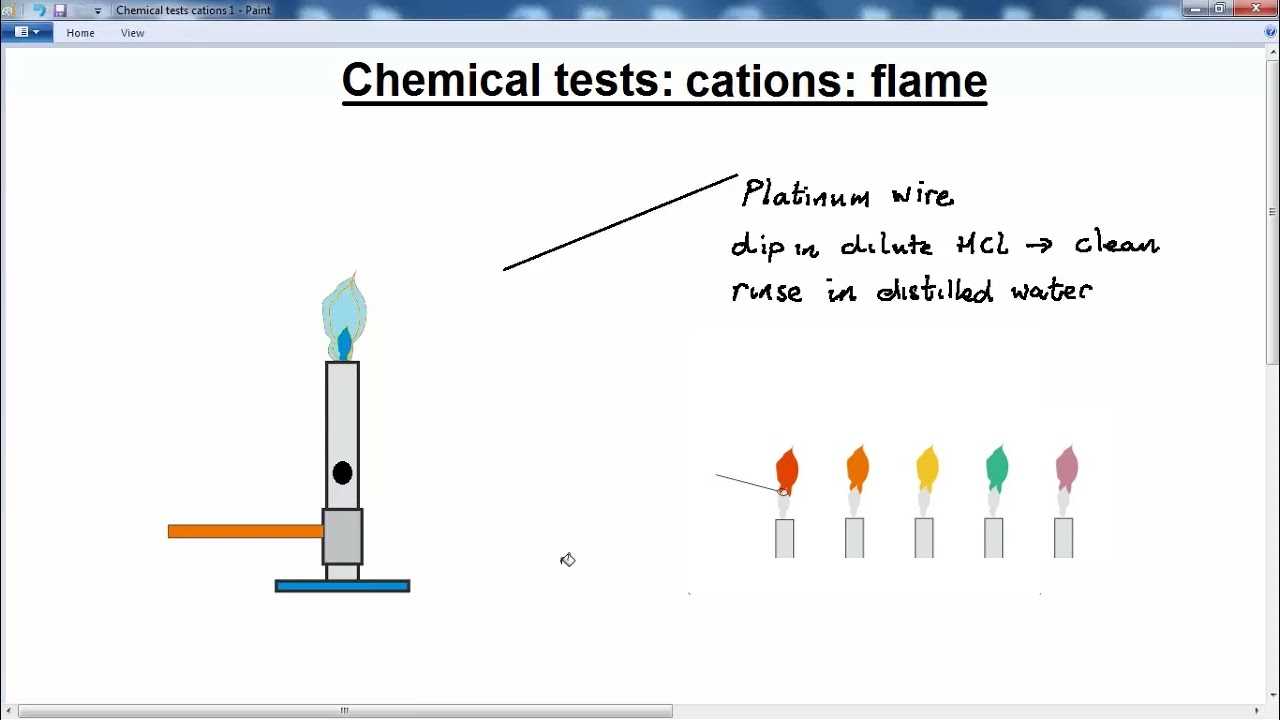
The process involves placing a sample of the compound in a controlled heat source, typically a Bunsen burner. Observing the emitted light is crucial for identifying the substances present. The intensity of the color and its consistency are important in distinguishing between compounds that might appear similar but react differently under heat.
Common Errors to Avoid
There are several factors that can impact the accuracy of the results:
- Contamination – Foreign substances can alter the color produced.
- Improper Heating – Uneven heating may prevent a proper reaction.
- Incorrect Sample Size – Too much or too little material can affect the intensity of the emitted light.
Applications in Chemistry
These reactions have numerous applications, from educational demonstrations to practical use in laboratories for identifying elements in unknown substances. They are often employed in fields such as forensic science, material analysis, and quality control in manufacturing.
Understanding the Reaction of Ions Under Heat

In this process, certain substances undergo reactions when exposed to high temperatures, producing distinct colors that can be used to identify their components. This technique relies on observing the light emitted by compounds when their atoms or ions absorb heat and release energy. The emitted light follows specific patterns, making it a valuable tool in chemical analysis.
Each type of ion, when heated, releases light at specific wavelengths, creating characteristic colors. These colors can be used to differentiate between various elements, making the method essential for the identification of compounds in various scientific fields.
Common Color Patterns in Reactions
The most common color changes observed include:
- Red – Associated with lithium and strontium.
- Blue – Seen in copper and cobalt.
- Green – Common with barium and copper.
- Yellow – Produced by sodium and potassium.
Setting Up the Experiment
To carry out this procedure, the substance is exposed to intense heat, typically from a Bunsen burner. The emitted light is carefully observed and compared with known standards for identification. Proper setup ensures that the reaction is observed clearly, and results are accurate.
Key Tools and Materials
Essential equipment for these experiments includes a heat source, such as a Bunsen burner, and clean wire loops or other holders to safely introduce the substance to the flame. Safety goggles and proper ventilation are also important to ensure a safe environment.
Analyzing and Interpreting Results
Once the reactions occur, analyzing the intensity and color of the light is crucial for drawing conclusions. Consistent patterns of color can confirm the presence of specific ions, while any unusual results may indicate contamination or improper procedure.