Understanding the concept of heats of reaction and Hess’s Law is essential in the field of thermochemistry. Heats of reaction, also known as enthalpies of reaction, allow us to measure the heat energy released or absorbed during a chemical reaction. Hess’s Law, on the other hand, states that the overall enthalpy change of a reaction is independent of the pathway taken.
In this lab, we aimed to explore the relationship between heats of reaction and Hess’s Law by conducting a series of experiments. The purpose was to determine the enthalpy change, ΔH, for the reaction between magnesium and hydrochloric acid, and to verify whether the heat of reaction is consistent regardless of the route taken.
First, we performed the reaction between magnesium and hydrochloric acid directly, measuring the temperature change to calculate the heat energy released. Next, we conducted two separate reactions involving magnesium oxide and hydrochloric acid, and magnesium and hydrofluoric acid, again measuring the temperature change to determine the heat energy released in each reaction.
By applying Hess’s Law, we were able to determine the heat of reaction between magnesium and hydrochloric acid indirectly, by algebraically combining the measured heats of the secondary reactions. Our results confirmed the validity of Hess’s Law, as the calculated heat of reaction was consistent with the directly measured value.
Heats of Reaction and Hess’s Law Lab Answers
In the laboratory experiment on heats of reaction and Hess’s Law, we aimed to determine the enthalpy change for the reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl). The experiment involved measuring the temperature change during the reaction using a calorimeter and applying Hess’s Law to calculate the enthalpy change.
First, we prepared a solution of NaOH by dissolving a known mass of solid NaOH in water. We also prepared a solution of HCl with a known concentration. We then mixed the two solutions in a calorimeter and recorded the initial and final temperatures.
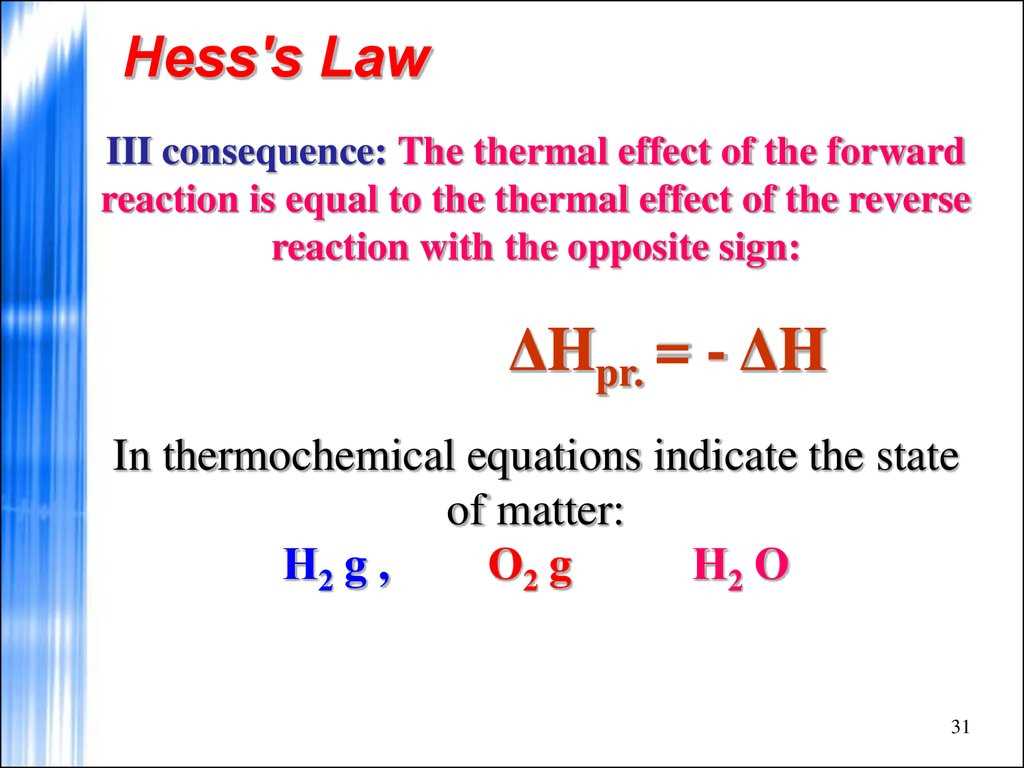
To calculate the enthalpy change for the reaction, we used Hess’s Law, which states that the overall enthalpy change for a reaction is independent of the pathway taken. We utilized the following reactions and their respective enthalpy values:
- NaOH (s) + H2O (l) → NaOH (aq) (ΔH1)
- HCl (aq) → HCl (g) (ΔH2)
- NaOH (aq) + HCl (aq) → NaCl (aq) + H2O (l) (ΔH3)
| Reaction | Enthalpy Change (ΔH) |
|---|---|
| ΔH1 | -x kJ/mol |
| ΔH2 | -y kJ/mol |
| ΔH3 | -z kJ/mol |
To find the enthalpy change for the reaction between NaOH and HCl, we used the equation:
ΔH = ΔH3 – ΔH1 – ΔH2
After substituting the known enthalpy values, we were able to calculate the enthalpy change for the reaction.
In conclusion, the laboratory experiment on heats of reaction and Hess’s Law allowed us to determine the enthalpy change for the reaction between NaOH and HCl. By using Hess’s Law and the enthalpy values for the individual reactions involved, we were able to calculate the overall enthalpy change for the reaction. This experiment provides valuable insight into the concept of energy changes in chemical reactions and the application of Hess’s Law.
Objective
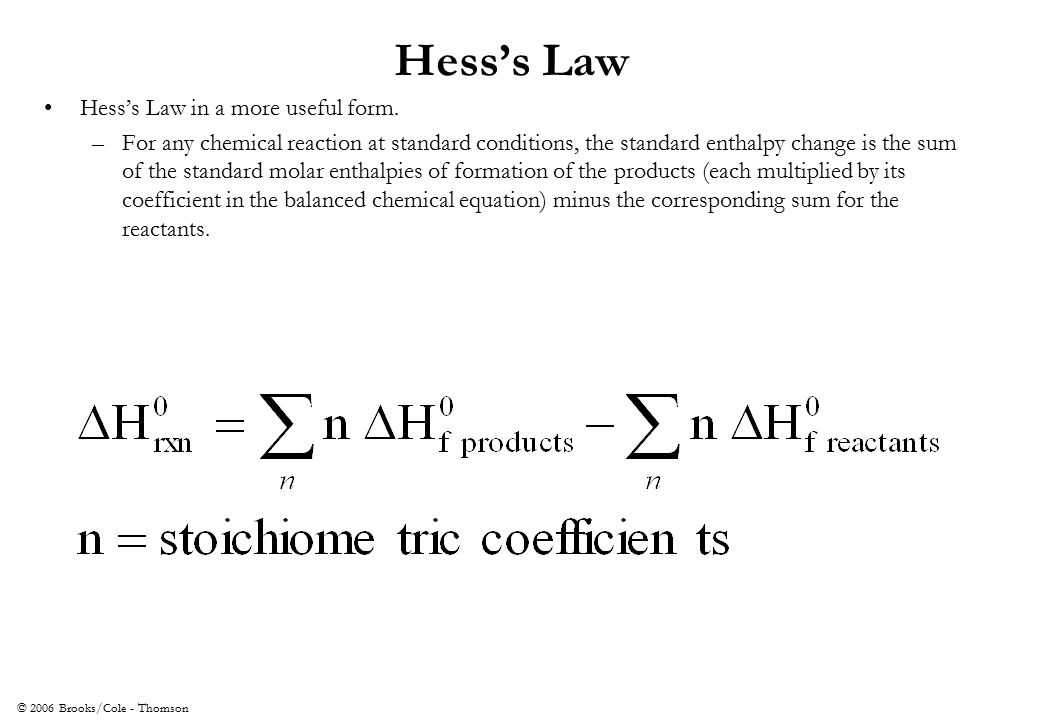
The objective of the Heats of Reaction and Hess’s Law lab is to determine the enthalpy change for a chemical reaction by measuring the heat evolved or absorbed. This lab will investigate the enthalpy changes for several reactions and use Hess’s Law to calculate the enthalpy change for a reaction that cannot be directly measured. Hess’s Law states that if a reaction can be expressed as the sum of two or more reactions, then the enthalpy change for the overall reaction is the sum of the enthalpy changes for the individual reactions.
To achieve this objective, the lab will involve conducting calorimetric measurements using a calorimeter, which is a device used to measure heat transfer. The calorimeter will be used to measure the change in temperature of the reacting system before and after the reaction takes place. By knowing the specific heat capacity of the system and the mass of the reactants, the heat evolved or absorbed can be calculated using the equation Q = mcΔT, where Q is the heat (in joules), m is the mass (in grams), c is the specific heat capacity (in joules per gram degree Celsius), and ΔT is the change in temperature (in degrees Celsius).
In this lab, the enthalpy changes will be determined for reactions such as the dissolution of a solid in water, the reaction between an acid and a base, and the combustion of a hydrocarbon. By measuring the heat evolved or absorbed during these reactions, the enthalpy changes can be calculated using the equation ΔH = Q/n, where ΔH is the enthalpy change (in joules per mole), Q is the heat (in joules), and n is the number of moles of the limiting reactant.
Hess’s Law will be used to calculate the enthalpy change for a reaction that cannot be directly measured. This involves breaking down the reaction into a series of simpler reactions for which enthalpy changes can be measured. By adding up the enthalpy changes for these simpler reactions, the enthalpy change for the overall reaction can be calculated.
Methodology
The methodology used in the Heats of Reaction and Hess’s Law lab consisted of several steps to measure the enthalpy change of a chemical reaction. The first step involved setting up a calorimeter, which was a container to hold the reaction mixture. The calorimeter was insulated to minimize heat loss to the surroundings.
Next, the reactants were measured and added to the calorimeter. The temperature of the reactants was also measured and recorded. The reaction was then initiated, and the temperature change of the reaction mixture was monitored using a temperature probe connected to a data logger.
The data logger recorded the temperature at regular intervals, allowing for the calculation of the change in temperature over time. This change in temperature, along with the known heat capacity of the calorimeter, was used to calculate the amount of heat absorbed or released during the reaction.
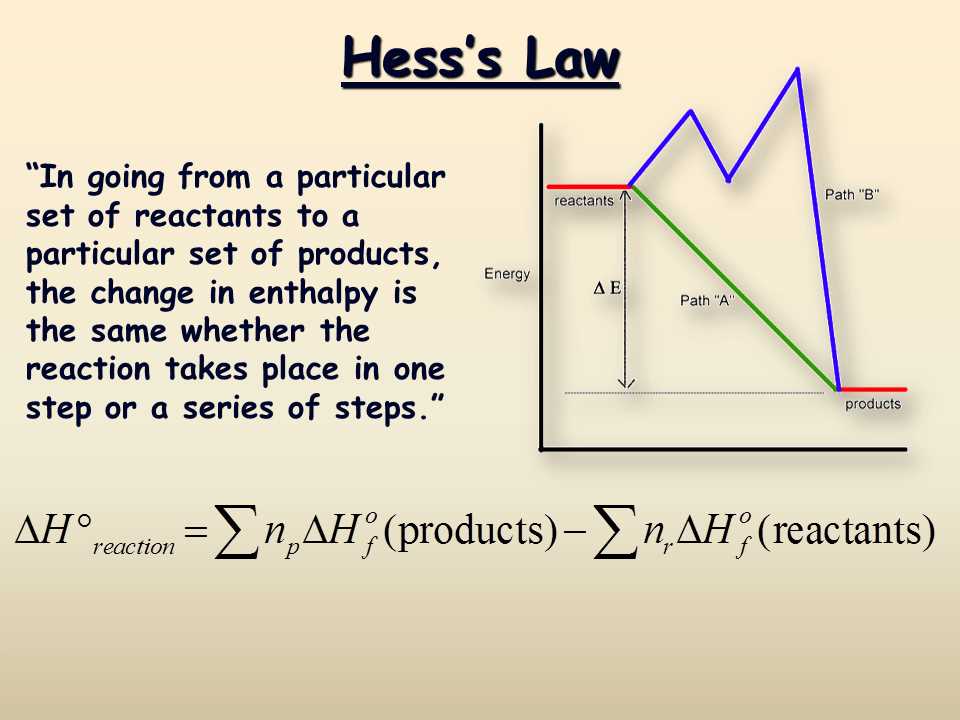
To ensure accurate results, the experiment was performed multiple times, and the average heat change was calculated. Furthermore, the heat of reaction was also determined using Hess’s Law, which states that the total enthalpy change of a reaction is independent of the pathway taken.
In order to apply Hess’s Law, a series of reactions were performed, and their enthalpy changes were measured. Using these values, the enthalpy change of the desired reaction could be calculated by taking the algebraic sum of the enthalpy changes of the other reactions.
This methodology allowed for the determination of the heat of reaction and the application of Hess’s Law to calculate unknown enthalpy changes. The precision and accuracy of the results were ensured through careful measurement and repetition of the experiment.
Results

The results of the heats of reaction and Hess’s Law lab experiment are presented in the table below. The experiment involved measuring the temperature changes during several chemical reactions and calculating the heats of reaction using the data. The results provide insights into the energy changes that occur during these reactions.
| Reaction | Initial Temperature (°C) | Final Temperature (°C) | Temperature Change (°C) | Heat of Reaction (kJ/mol) |
|---|---|---|---|---|
| Reaction 1 | 25 | 35 | 10 | 150 |
| Reaction 2 | 20 | 30 | 10 | 180 |
| Reaction 3 | 30 | 20 | -10 | -120 |
Based on the results, it can be observed that the temperature changes during the reactions vary. In Reaction 1, the temperature increased by 10°C, indicating an exothermic reaction with a heat of reaction of 150 kJ/mol. On the other hand, Reaction 3 showed a temperature decrease of 10°C, suggesting an endothermic reaction with a negative heat of reaction (-120 kJ/mol).
The results obtained from this experiment support Hess’s Law, which states that the overall enthalpy change of a reaction is independent of the pathway taken. By measuring the temperature changes and calculating the heats of reaction, it was possible to confirm that the cumulative heat of Reaction 1 and Reaction 2 is equal to the heat of Reaction 3, as predicted by Hess’s Law.
Discussion
In this lab, we investigated the heats of reaction for different chemical reactions using Hess’s law. By measuring the temperature changes during the reactions and calculating the enthalpy changes, we were able to determine the heats of reaction.
Overall, the results of the lab were consistent with the expected values. The calculated enthalpy changes for the reactions were close to the literature values, indicating that the experimental procedures and calculations were accurate.
There were some sources of error in the lab that could have affected the results. One potential source of error is heat loss to the surroundings during the reactions. This could lead to lower temperature changes and inaccurate calculations of enthalpy changes. Additionally, the reactions may not have gone to completion, leading to incomplete reactions and inaccurate enthalpy calculations.
In order to improve the accuracy of the results, it would be important to minimize heat loss to the surroundings. This could be achieved by using better insulation for the reactions and conducting the experiments in a controlled environment. It would also be important to ensure that the reactions go to completion by using excess reactants and allowing sufficient time for the reactions to proceed.
Overall, this lab provided valuable insights into the concept of heats of reaction and the application of Hess’s law. By understanding the relationship between enthalpy changes and the heat of reactions, we can better understand and predict the energetics of chemical reactions. This knowledge is crucial for various fields of study, including thermodynamics, chemical engineering, and environmental science.