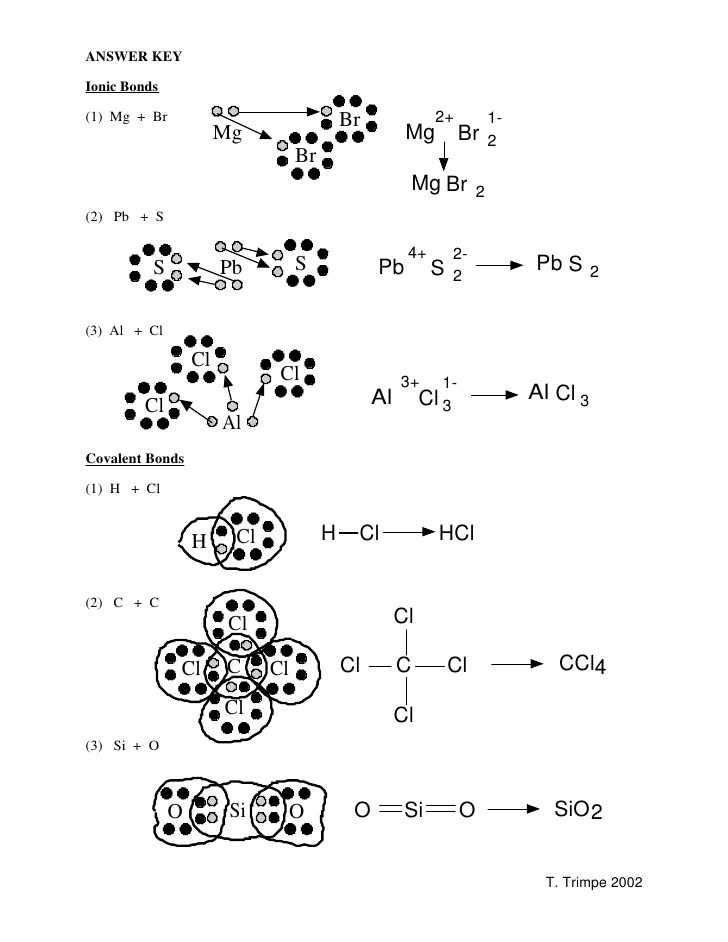
Ionic bonds are a type of chemical bond characterized by the electrostatic attraction between positively and negatively charged ions. This type of bond typically forms between a metal and a non-metal, where the metal donates one or more electrons to the non-metal, resulting in the formation of positively and negatively charged ions.
In the POGIL activity on ionic bonds, students are presented with a series of questions and guided through the process of answering them. The answer key for this activity provides students with the correct answers to these questions, allowing them to compare their own responses and further their understanding of the concept.
The answer key also includes detailed explanations for each answer, helping students grasp the underlying principles and concepts behind ionic bonds. This is crucial for building a solid foundation of knowledge in chemistry and understanding the role of ionic bonds in various chemical reactions and processes.
By using the Ionic Bonds POGIL answer key, students are able to assess their own understanding of the topic and identify areas where they may need further study or clarification. It serves as a valuable tool for both students and educators in promoting active learning and facilitating the acquisition of knowledge and skills related to ionic bonds.
Ionic Bonds POGIL Answer Key: Exploring the Fundamental Concepts
In the study of chemistry, understanding the fundamental concepts of ionic bonds is crucial. Ionic bonds occur between atoms when one atom transfers electrons to another atom. This transfer creates ions with opposite charges, which are then attracted to each other and form a bond.
The Ionic Bonds POGIL (Process Oriented Guided Inquiry Learning) answer key explores various aspects of ionic bonding. It provides students with a comprehensive understanding of the key concepts, including how to determine the charges of ions, how to write formulas for ionic compounds, and how to name these compounds using the rules of nomenclature.
Determining the Charges of Ions:
- The answer key helps students understand how to determine the charges of ions based on the location of the element on the periodic table. For example, elements in Group 1A (such as sodium) typically form ions with a +1 charge, while elements in Group 7A (such as chlorine) typically form ions with a -1 charge.
- Students are also guided through the process of determining charges for transition metals, which can have multiple charges.
Writing Formulas for Ionic Compounds:
- The answer key provides examples and step-by-step instructions on how to write formulas for ionic compounds. This involves balancing the charges of the ions and ensuring that the overall charge of the compound is neutral.
- Students learn how to use subscripts to indicate the ratio of ions in the compound and how to simplify the formula if necessary.
Naming Ionic Compounds:
- Using the answer key, students learn the rules of nomenclature for naming ionic compounds. This includes identifying the cation and anion in the compound and combining their names, along with appropriate suffixes and prefixes if necessary.
- Students also learn how to differentiate between compounds with different charges for the same element, using Roman numerals to indicate the charge of the cation.
The Ionic Bonds POGIL answer key serves as a valuable resource for students to reinforce their understanding of these fundamental concepts. By providing clear explanations and step-by-step instructions, it helps students develop the skills necessary to solve complex problems related to ionic bonding.
Overall, the answer key helps students gain a solid foundation in the principles of ionic bonds, setting them up for success in their further studies of chemistry.
Understanding Ionic Bonds: Definition and Formation
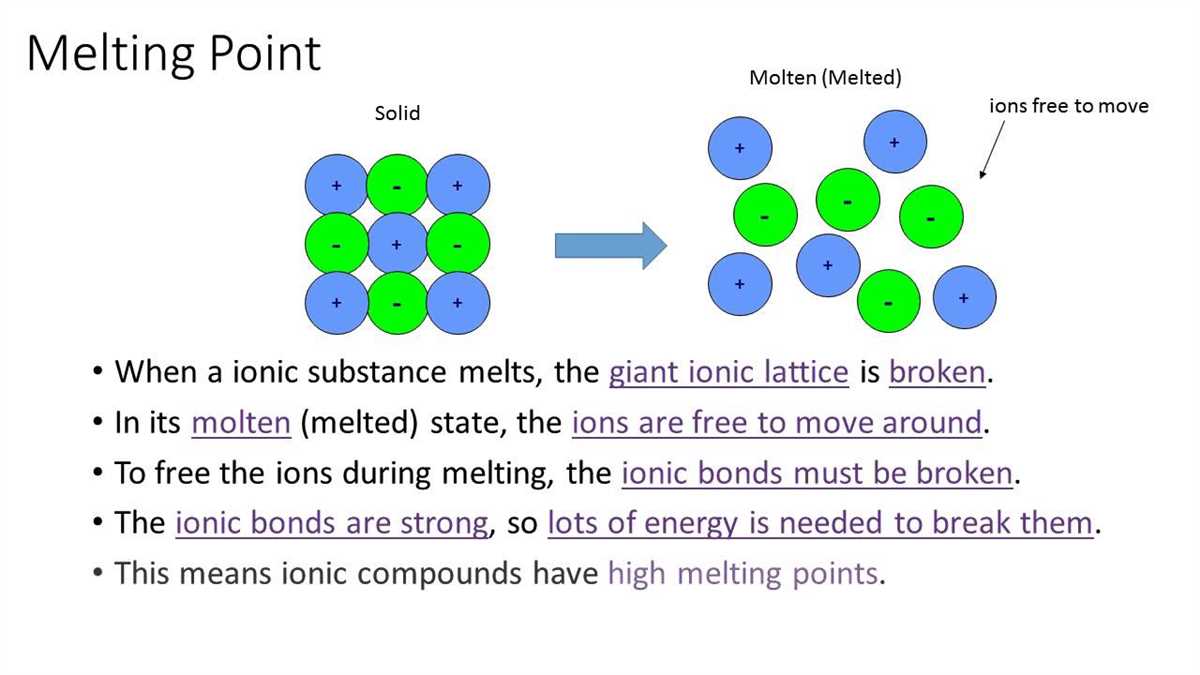
An ionic bond is a type of chemical bond that occurs between atoms when one or more electrons are transferred from one atom to another. This transfer of electrons results in the formation of positively charged ions (cations) and negatively charged ions (anions). Ionic bonds typically occur between metals and nonmetals, where the metal atom loses electrons to become a cation and the nonmetal atom gains electrons to become an anion.
The formation of an ionic bond involves a process called ionization, where electrons are either gained or lost by atoms. This process occurs due to the difference in electronegativity between the atoms involved. Electronegativity is a measure of the tendency of an atom to attract electrons towards itself. When metals and nonmetals combine, the metal atom has a lower electronegativity and therefore tends to lose electrons, while the nonmetal atom has a higher electronegativity and tends to gain electrons.
In an ionic bond, the positive and negative ions are attracted to each other due to their opposite charges. The attraction between these ions is strong and typically results in the formation of a crystalline solid, such as sodium chloride (NaCl) or calcium carbonate (CaCO₃). These ionic compounds have a regular repeating pattern of positive and negative ions in a three-dimensional lattice structure.
In summary, ionic bonds occur when electrons are transferred between atoms with different electronegativities. This transfer results in the formation of positively charged cations and negatively charged anions, which are then attracted to each other to form a stable ionic compound. Understanding the definition and formation of ionic bonds is essential for understanding the properties and behavior of numerous chemical compounds in various fields of science and industry.
The Octet Rule and Its Role in Ionic Bonding

The octet rule is a fundamental concept in chemistry that states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. This rule is based on the observation that atoms are most stable when their outer electron shell is filled with eight electrons, resembling the electron configuration of noble gases. The octet rule is particularly important in understanding the formation of ionic bonds.
Ionic bonding occurs between atoms when one atom transfers electrons to another, resulting in the formation of oppositely charged ions. The atom that loses electrons becomes a positively charged ion, called a cation, while the atom that gains electrons becomes a negatively charged ion, called an anion. The transfer of electrons allows both ions to achieve a stable electron configuration of eight valence electrons, satisfying the octet rule.
For example, in the formation of sodium chloride (NaCl), sodium (Na) loses one electron to become a positively charged sodium ion (Na+), while chlorine (Cl) gains one electron to become a negatively charged chloride ion (Cl-). The attraction between the oppositely charged ions results in the formation of an ionic bond.
The octet rule provides a framework for predicting the formation of ionic compounds and understanding their properties. Ionic compounds generally have high melting and boiling points, are crystalline solids, and are good conductors of electricity in the molten or aqueous state. These properties can be attributed to the strong electrostatic attraction between the positively and negatively charged ions in the crystal lattice.
In summary, the octet rule plays a crucial role in ionic bonding by guiding the transfer of electrons and ensuring the stability of the resulting ions. Understanding the octet rule helps explain the formation and properties of ionic compounds, contributing to our overall understanding of chemical bonding and reactivity.
Electronegativity and Ionic Bonds: The Key Factors
The concept of electronegativity plays a crucial role in understanding ionic bonds. Electronegativity refers to the ability of an atom to attract electrons towards itself when it is bonded with another atom. The electronegativity difference between two atoms determines the degree of polarity in a chemical bond. In ionic bonding, the electronegativity difference is often significant, resulting in the transfer of electrons from one atom to another.
The key factor in the formation of ionic bonds is the significant difference in electronegativity between the atoms involved. Typically, ionic bonds occur between a metal and a nonmetal element, where the metal is a cation (positively charged ion) and the nonmetal is an anion (negatively charged ion). The transfer of electrons from the metal to the nonmetal creates a bond between the oppositely charged ions, resulting in the formation of an ionic compound.
In an ionic bond, the atom with higher electronegativity attracts the electrons more strongly and gains a negative charge, while the atom with lower electronegativity loses electrons and gains a positive charge. This transfer of electrons creates a strong electrostatic attraction between the positive and negative ions, holding them together in a solid crystal lattice structure.
The formation of ionic bonds is influenced by the electronegativity difference between atoms, as well as other factors such as the size of the atoms and the availability of electron orbitals. The greater the electronegativity difference, the stronger the ionic bond will be. Ionic compounds typically have high melting and boiling points due to the strong electrostatic forces between the ions.
In conclusion, understanding the concept of electronegativity and its role in the formation of ionic bonds is crucial for explaining the properties and behavior of ionic compounds. The significant electronegativity difference between atoms leads to the transfer of electrons and the formation of oppositely charged ions, resulting in a strong bond between the ions.
POGIL Activity: Unraveling Ionic Bonding and Compound Formation
In this POGIL activity, students will investigate the concept of ionic bonding and compound formation. Ionic bonding is a type of chemical bond that occurs between ions with opposite charges. It involves the transfer of electrons from one atom to another, resulting in the formation of stable compounds.
This activity begins with a focus on the basic properties of ions and their charges. Students will identify the charges of various ions and understand how they interact to form compounds. Through guided inquiry, they will explore the rules for predicting the charges of monatomic ions and understand the concept of electronegativity.
Next, the activity delves into the process of ionic compound formation. Students will learn about the role of cations and anions in creating stable compounds. They will analyze the charges and ratios of ions in different compounds to determine their formulas and names. Through this process, students will gain a deeper understanding of the rules and patterns governing ionic bonding.
A variety of POGIL worksheets, including diagrams, tables, and fill-in-the-blank exercises, will guide students through this activity. These worksheets will prompt them to analyze and interpret data, make predictions, and engage in collaborative learning. By actively participating in this activity, students will develop a solid foundation in understanding the nature of ionic bonds and the formation of compounds.
Common Examples of Ionic Compounds in Everyday Life
Ionic compounds are a common type of chemical compound that form when atoms gain or lose electrons to achieve a stable electron configuration. These compounds have a wide range of practical applications and can be found in many everyday items that we use.
Table Salt (Sodium Chloride): One of the most well-known examples of an ionic compound is table salt, or sodium chloride. It is made up of sodium cations and chloride anions, which are held together by strong ionic bonds. Table salt is used for seasoning food, preserving food, and as a natural remedy for various health conditions.
Baking Soda (Sodium Bicarbonate): Another commonly used ionic compound is baking soda, or sodium bicarbonate. It is formed by the reaction of sodium hydroxide and carbon dioxide, and it is often used as a leavening agent in baking. Baking soda also has various other uses, such as cleaning, odor absorption, and as a mild antacid.
Calcium Carbonate: Calcium carbonate is an ionic compound that is found in many natural materials, such as limestone, marble, and chalk. It is also commonly used as an ingredient in toothpaste, antacids, and calcium supplements. Calcium carbonate provides strength and stability to bones and teeth, and it is also used as a filler in many products, such as paint and plastics.
Potassium Nitrate: Potassium nitrate is an ionic compound that is used in various industries, including agriculture and pyrotechnics. It is often used as a fertilizer and as an oxidizing agent in fireworks. Potassium nitrate also has medical applications, such as in the treatment of high blood pressure and as a component of some toothpastes.
Magnesium Sulfate: Magnesium sulfate, also known as Epsom salt, is an ionic compound that is commonly used in bath salts and foot soaks for its soothing and relaxing properties. It can also be used as a fertilizer, a laxative, and as a treatment for certain medical conditions, such as magnesium deficiency and preterm labor.
These are just a few examples of the many ionic compounds that are used in everyday life. From table salt to Epsom salt, these compounds play important roles in various industries and provide us with a wide range of benefits and applications.
Practical Applications and Significance of Ionic Bonds
Ionic bonds have numerous practical applications in various fields and play a crucial role in our everyday lives. Some of the key areas where ionic bonds are significant include:
- Chemical Reactions: Ionic bonds are essential in many chemical reactions. They help in the formation of stable compounds and facilitate the transfer of electrons between atoms.
- Electricity Generation: Ionic compounds, such as table salt (sodium chloride), are used in electrolyte solutions for the generation of electricity in batteries. The movement of ions between the positive and negative electrodes enables the flow of electrons, producing electrical energy.
- Industrial Processes: Many industrial processes rely on the use of ionic compounds. For example, the production of aluminum involves the extraction of aluminum ions from bauxite ore using electrolysis.
- Medicine and Pharmaceuticals: Ionic bonds are important in the field of medicine and pharmaceuticals. Many drugs are formulated as ionic salts to improve their solubility and enhance their efficacy.
- Crystal Structures: Ionic bonds are responsible for the formation of the crystal lattice structures of many minerals and gemstones, giving rise to their unique physical properties and characteristics.
The significance of ionic bonds extends beyond these applications. They serve as the foundation for understanding and explaining the behavior of many chemical compounds and materials. Additionally, the study of ionic bonds contributes to the development of new materials, advancements in technology, and the overall progress of scientific research.
In conclusion, ionic bonds are of great practical importance and have a significant impact on various aspects of our lives. Their role in chemical reactions, electricity generation, industrial processes, medicine, and crystal structures highlights their significance in different fields. Understanding and harnessing the power of ionic bonds continues to drive innovation and contribute to advancements in various scientific and technological domains.