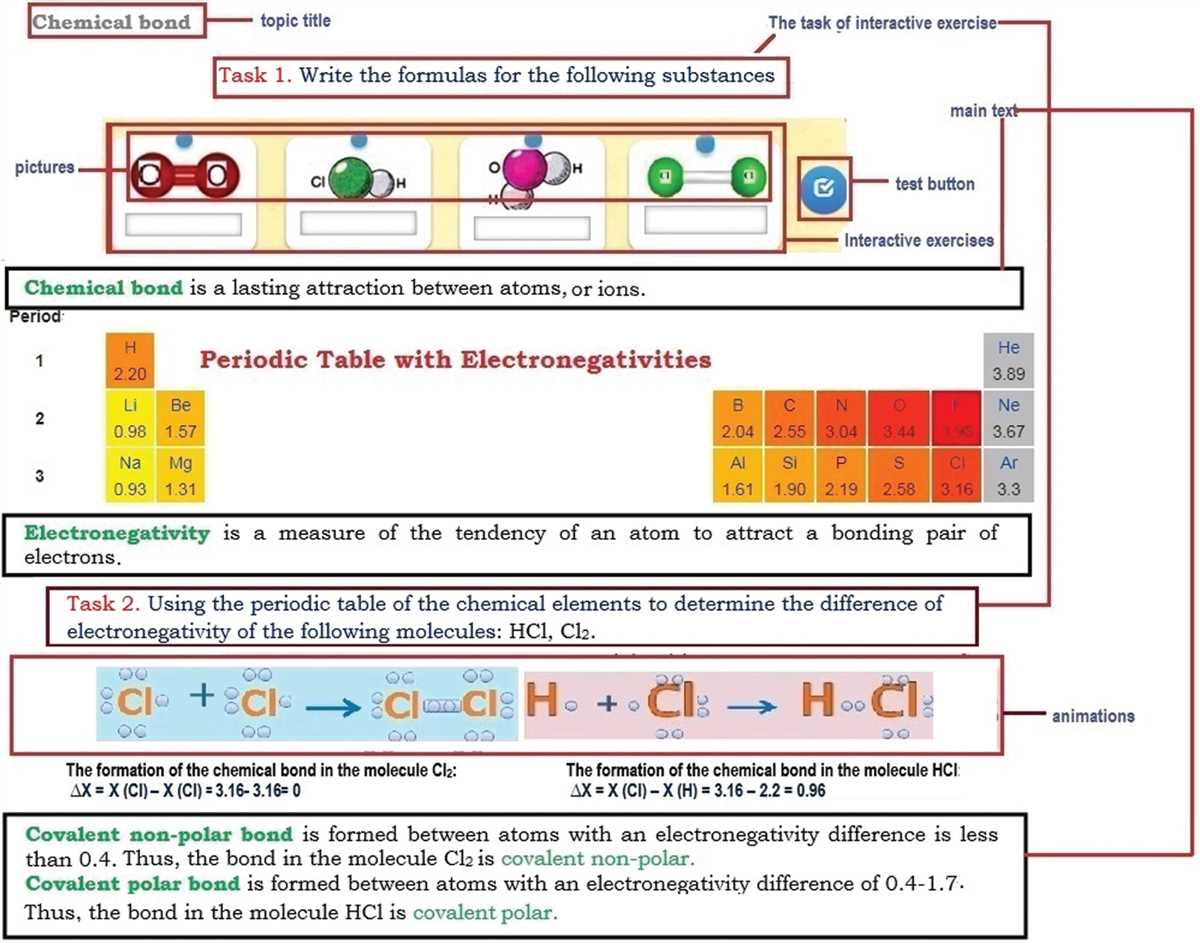
Understanding the concept of molecular polarity is crucial in the field of chemistry. Whether it is determining the nature of a compound or predicting its physical and chemical properties, knowing the polarity of molecules plays a significant role in scientific research. The Molecule Polarity PhET Lab is an interactive virtual simulation that allows students to explore the concept of molecular polarity and understand its importance in various chemical reactions.
In this lab, students are introduced to the concept of polar and nonpolar molecules and are given the opportunity to manipulate different molecules to observe their polarity. The lab starts with a brief introduction to the definition of polarity and its impact on the behavior of different substances. It goes on to explain the factors that determine the polarity of a molecule, including the presence of polar bonds and the molecular geometry.
The PhET simulation provides students with a virtual lab environment where they can experiment with different molecules and observe the effect of changing the molecular structure on its polarity. By dragging various atoms and bonds, students can build different molecules, and the simulation instantly calculates and displays the polarity of the molecule. This hands-on experience allows students to develop an intuitive understanding of the concept and enhances their analytical skills.
The Molecule Polarity PhET Lab also includes a set of questions and activities that reinforce the learned concepts and test the students’ understanding. By answering these questions, students can demonstrate their knowledge of molecular polarity and its implications in chemical reactions. Additionally, the lab provides an answer key, which serves as a valuable resource for students to verify their answers and learn from their mistakes.
Molecule Polarity Phet Lab Answer Key
In the Molecule Polarity Phet Lab, students are introduced to the concept of polarity in molecules. They explore the factors that contribute to a molecule’s polarity, such as the individual bond polarities and the molecular geometry. Understanding molecule polarity is important as it affects the physical and chemical properties of substances, such as their solubility and reactivity.
To determine the polarity of a molecule, students use a virtual lab provided by Phet Interactive Simulations. This lab allows them to build and visualize different molecules with varying bond polarities and molecular geometries. By dragging and dropping atoms and adjusting their positions, students can construct molecules and observe their polarity in real-time.
The Molecule Polarity Phet Lab Answer Key provides students with the correct answers to the lab questions and helps them check their understanding of the concepts covered in the lab. It serves as a reference guide for students to compare their own observations and conclusions with the expected outcomes. Additionally, the answer key can be used by teachers to facilitate discussions and explanations during class.
Overall, the Molecule Polarity Phet Lab and its answer key are valuable educational resources that enhance students’ understanding of molecular polarity and its implications in chemistry. By engaging in virtual experiments and analyzing their results, students can develop a deeper comprehension of this fundamental concept in chemistry.
Overview
The Molecule Polarity PhET Lab is an interactive simulation designed to help students understand the concept of polarity in molecules. In this lab, students are able to explore the behavior of different molecules and determine their polarity based on the arrangement of the atoms and the distribution of electron density.
The lab begins with an introduction to the concept of polarity and its importance in determining the physical and chemical properties of molecules. Students are then able to manipulate the atoms and bonds in different molecules to observe how changes in structure affect molecular polarity.
The simulation provides students with a visual representation of the electron distribution within molecules, allowing them to see how the arrangement of atoms and their associated electronegativities can result in a molecule being polar or nonpolar. Students can also measure the dipole moment of different molecules to further understand the relationship between molecular structure and polarity.
The Molecule Polarity PhET Lab is a valuable tool for teaching students about the concept of polarity and its impact on molecular behavior. By allowing students to interact with the simulation and analyze different molecules, they are able to develop a deeper understanding of the factors that contribute to molecular polarity.
Background

In this lab, we will explore the concept of molecule polarity using the PhET interactive simulation. Molecules can be either polar or nonpolar depending on the distribution of charge within them. This distribution is determined by the arrangement of atoms and the electronegativity difference between them.
Electronegativity is a measure of an atom’s ability to attract electron density towards itself in a covalent bond. When atoms in a molecule have different electronegativities, a partial positive and a partial negative charge are created on the atoms, resulting in a polar molecule. If the electronegativity difference between the atoms is small or zero, the molecule is nonpolar.
In this lab, we will investigate the factors that determine the polarity of molecules. We will explore how the shape and symmetry of a molecule affect its polarity, as well as the role of electronegativity. We will use the PhET simulation to visualize and manipulate different molecules, and observe the resulting changes in polarity.
Procedure
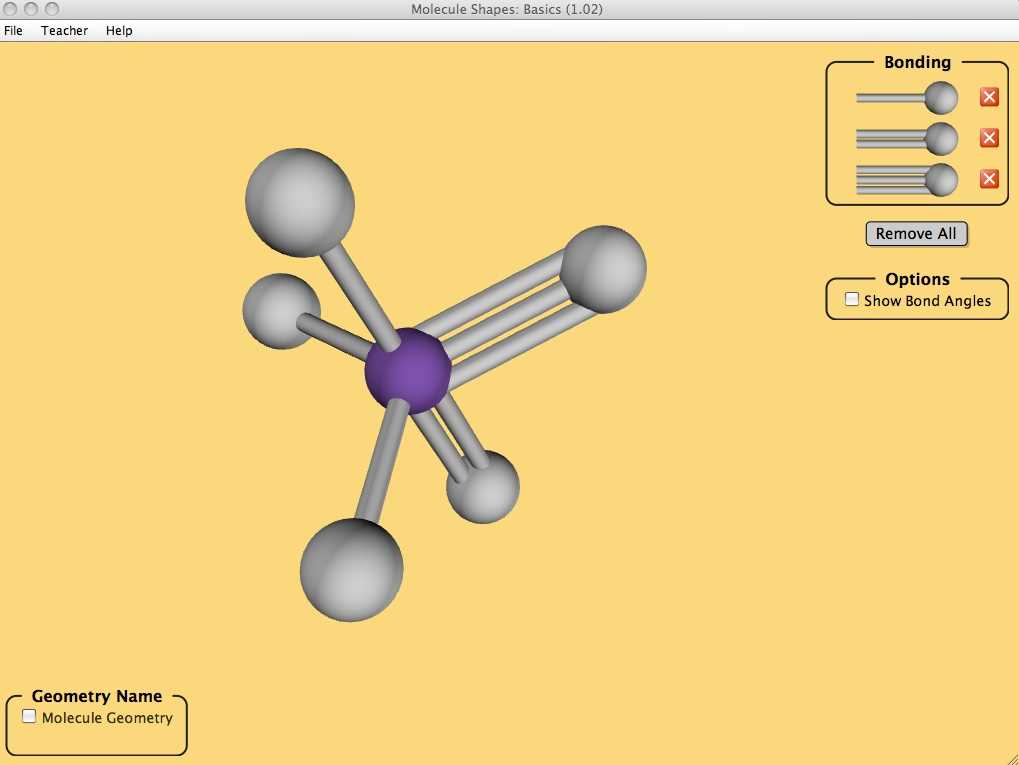
In this lab, you will be using the PhET simulation “Molecule Polarity” to investigate the polarity of different molecules. Follow the steps below to complete the lab:
- Open the PhET simulation “Molecule Polarity” on your computer.
- Observe the different molecular structures that are displayed on the screen.
- Click on each molecule to rotate it and view it from different angles.
- Use the “Polarity” button to check if the molecule is polar or nonpolar.
- Record your observations and data in a table, noting if the molecule is polar or nonpolar.
- Repeat steps 2-5 for all of the molecules provided.
- Once you have observed all of the molecules, analyze your data to identify any patterns or trends.
- Write a conclusion summarizing your findings and explaining the factors that contribute to molecule polarity.
Remember to refer to the provided answer key to check your answers and ensure accuracy in your observations and data recording.
Results
In this lab, we observed the polarity of various molecules using the Molecule Polarity PhET simulation. We used the simulation to investigate the properties of different molecules and determine whether they were polar or nonpolar.
By manipulating the atoms and bonds in the simulation, we were able to see how the shape and distribution of charge in a molecule affect its polarity. We found that molecules with an uneven distribution of charge, or a polar bond, were considered polar, while molecules with an even distribution of charge, or a nonpolar bond, were considered nonpolar.
During the lab, we discovered that molecular shape plays a significant role in determining whether a molecule is polar or nonpolar. For example, molecules with a symmetrical shape, such as methane (CH4), were nonpolar, while molecules with an asymmetrical shape, such as water (H2O), were polar.
Additionally, electronegativity also influenced the polarity of a molecule. If the difference in electronegativity between the atoms in a bond was significant, the bond would be polar, resulting in a polar molecule. For example, in the case of hydrogen chloride (HCl), the electronegativity difference between hydrogen and chlorine caused the molecule to be polar.
In conclusion, the Molecule Polarity PhET lab provided a hands-on way to explore the concept of molecular polarity. It allowed us to visualize and manipulate different molecules, understand how their shape and electronegativity contribute to their polarity, and ultimately determine whether they are polar or nonpolar. This lab served as a valuable tool in reinforcing our understanding of the topic and allowed us to apply our knowledge in a practical and interactive manner.
Q&A:
What are results?
Results are outcomes or consequences that occur as a result of certain actions, events, or decisions.
Why are results important?
Results are important because they provide information about the effectiveness or success of a particular task, project, or endeavor. They help in evaluating performance and making decisions for future actions.
How can I improve my results?
To improve your results, you can set clear goals, develop a plan, prioritize tasks, stay focused, seek feedback, and continuously learn and adapt to new situations.
What should I do if I’m not satisfied with the results?
If you are not satisfied with the results, you can analyze the reasons behind the outcome, identify areas for improvement, and make necessary adjustments. It may also be helpful to seek advice or assistance from others who have expertise or experience in the particular field.
How can I measure the results?
You can measure results by setting specific, measurable, achievable, relevant, and time-bound (SMART) goals. By comparing the actual outcomes or achievements with the predetermined goals, you can determine the level of success or progress.
What are results?
Results are the outcomes or consequences of an action or event. They can be positive or negative, depending on the desired outcome and the effectiveness of the action taken.