If you are studying chemistry, chances are you have come across the topic of stoichiometry. Stoichiometry is the study of the quantitative relationship between reactants and products in a chemical reaction. It is an essential concept in chemistry as it allows us to predict the amount of products formed from a given amount of reactants, and vice versa.
However, stoichiometry can be a challenging topic for many students. The Pogil stoichiometry packet, with its set of practice problems and corresponding answers, is a valuable resource that can help students understand and master this concept.
In this article, we will provide you with a comprehensive guide to the Pogil stoichiometry packet answers. We will break down the different types of problems included in the packet and explain the step-by-step process for solving them. Whether you are struggling with mole-mole conversions, mass-mass conversions, or limiting reactant problems, you will find all the answers and explanations you need right here.
Pogil Stoichiometry Packet Answers
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It involves using mathematical calculations to determine the amount of reactants needed, the amount of products produced, and other related quantities. Pogil stands for Process Oriented Guided Inquiry Learning, which is a teaching method that encourages students to work collaboratively and actively engage in the learning process.
The Pogil Stoichiometry Packet is a resource that provides students with guided inquiry activities and practice problems to help them understand and apply the principles of stoichiometry. The packet includes questions and prompts that require students to analyze and interpret chemical equations, balance equations, calculate stoichiometric ratios and amounts, and solve stoichiometry problems using mole-mole, mole-mass, and mass-mass relationships.
Answering the Pogil Stoichiometry Packet
When answering the Pogil Stoichiometry Packet, it is important to carefully read and understand each question or prompt. Start by analyzing the given chemical equation and identifying the reactants and products involved. Then, determine the stoichiometric ratio between the reactants and products by balancing the equation if necessary. Use the coefficients in the balanced equation to convert between moles of the given substance and moles of the desired substance.
Next, use the stoichiometric ratio and the given quantity of one substance to calculate the unknown quantity of another substance. This can be done using mole-mole, mole-mass, or mass-mass calculations, depending on the information given. Be sure to properly set up the conversion factors and units to cancel out the unwanted units and obtain the desired units in the final answer.
It is recommended to show all the steps and calculations involved in solving each problem in a clear and organized manner. This not only helps in understanding the solution process but also allows for easy identification of any errors made. Additionally, it is important to check the units and significant figures in the final answer to ensure accuracy and precision.
What is Stoichiometry?
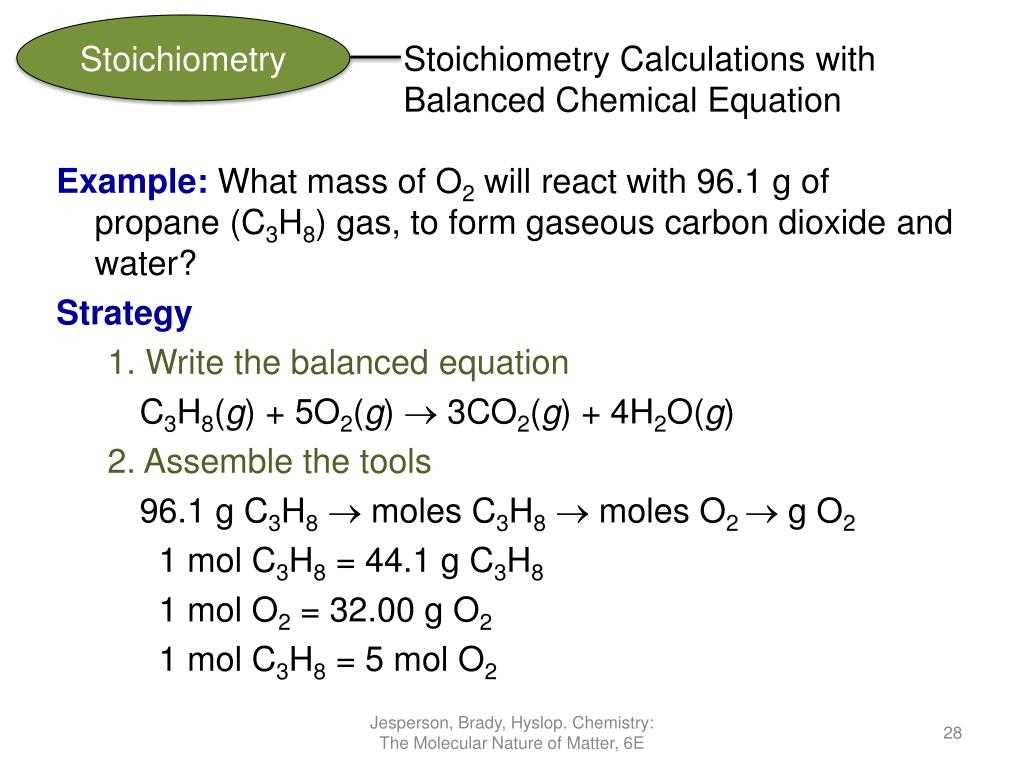
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between the reactants and products in chemical reactions. It involves the calculation of the mass, volume, or number of particles involved in a chemical reaction, based on the balanced equation for the reaction.
In stoichiometry, the concept of a mole is fundamental. A mole represents a fixed number of particles, which is approximately equal to 6.022 x 10^23 particles. This number is known as Avogadro’s number. Using the concept of moles, stoichiometry allows us to determine the amount of one substance that is required to react with or produce a certain amount of another substance.
The stoichiometric calculations are based on the stoichiometric coefficients in a balanced chemical equation. These coefficients represent the ratio of moles of each substance involved in the reaction. By using the stoichiometric coefficients, we can calculate the amount of reactants needed or products formed in a chemical reaction.
Stoichiometry plays a crucial role in various areas of chemistry, including analytical chemistry, environmental chemistry, and industrial chemistry. It allows chemists to predict and control the outcome of chemical reactions, optimize reaction conditions, and determine the purity of products. Overall, stoichiometry is an essential tool for understanding and manipulating chemical reactions.
Why is Stoichiometry Important?
Stoichiometry is a fundamental concept in chemistry that plays a crucial role in determining the quantities of reactants and products in a chemical reaction. It allows us to understand the relationship between the mass, moles, and molar ratios of substances involved in a reaction. Stoichiometry helps scientists predict the amount of product that can be obtained from a given amount of reactants and vice versa.
One of the main reasons why stoichiometry is important is its application in the field of synthesis. By understanding stoichiometry, chemists can determine the precise amounts of reactants needed to produce a desired amount of product. This knowledge is invaluable in industries such as pharmaceuticals, where the synthesis of drugs requires careful control over the reactant ratios to ensure maximum yield and purity. Without stoichiometry, it would be challenging to scale up reactions or optimize reaction conditions.
Stoichiometry is also essential in determining the theoretical yield of a reaction, which represents the maximum amount of product that can be obtained under ideal conditions. By comparing the actual yield of a reaction to the theoretical yield, scientists can assess the efficiency of a reaction and make adjustments to improve its yield. This information is crucial in industrial processes, where optimizing yield is essential for cost-effectiveness.
Furthermore, stoichiometry provides insights into the stoichiometric ratios of reactants and products, allowing chemists to identify the limiting and excess reactants in a reaction. This knowledge helps in understanding the overall reaction mechanism and predicting the quantity of each reactant required for complete consumption. It also enables chemists to calculate the amounts of by-products or waste generated during a reaction, aiding in environmental considerations and waste management strategies.
In conclusion, stoichiometry is a fundamental concept with far-reaching applications in chemistry and related industries. It enables scientists to determine the quantity of reactants and products in a reaction, optimize reaction conditions, predict yields, and understand the overall reaction mechanism. Its importance lies in its ability to provide quantitative insights and guide decision-making in chemical synthesis, industrial processes, and environmental considerations.
Understanding Reactants and Products
One of the fundamental concepts in chemistry is understanding the relationship between reactants and products in a chemical reaction. Reactants are the substances that enter into a chemical reaction, while products are the substances that are formed as a result of the reaction. Reactants and products can be represented by chemical equations, where the reactants are written on the left side of the equation and the products are written on the right side.
In order to understand the reaction, it is important to know the stoichiometry, or the quantitative relationship, between the reactants and products. This is determined by the coefficients in the balanced chemical equation, which indicate how many molecules or moles of each reactant and product are involved in the reaction. By knowing the stoichiometry, scientists can determine the amount of reactants needed to produce a certain amount of products, or vice versa.
For example:
- In the reaction 2H2 + O2 → 2H2O, hydrogen gas (H2) and oxygen gas (O2) are the reactants, while water (H2O) is the product. The coefficient of 2 in front of H2O indicates that two molecules of water are produced for every two molecules of hydrogen gas and one molecule of oxygen gas.
- In the reaction C6H12O6 → 2C2H5OH + 2CO2, glucose (C6H12O6) is the reactant, while ethanol (C2H5OH) and carbon dioxide (CO2) are the products. The coefficient of 2 in front of C2H5OH and CO2 indicates that two molecules of ethanol and two molecules of carbon dioxide are produced for every one molecule of glucose.
Understanding the relationship between reactants and products is essential for predicting the outcomes of chemical reactions, as well as for determining the efficiency and yield of a reaction. It also allows scientists to design and optimize chemical processes in various industries, such as pharmaceuticals, energy production, and materials science.
Calculating Mole Ratios
In stoichiometry, mole ratios are used to determine the relative amounts of substances involved in a chemical reaction. These ratios are derived from the balanced equation for the reaction, which shows the molar ratio between the reactants and the products. Mole ratios are important because they allow us to make predictions about the quantities of substances consumed and produced in a reaction.
To calculate a mole ratio, you first need to determine the balanced equation for the reaction. This equation shows how the reactants combine to form the products, and it includes coefficients that represent the relative number of moles of each substance involved. These coefficients can be used as mole ratios to convert between the different substances in the reaction.
For example, let’s consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). The balanced equation for this reaction is:
| 2H2 + O2 → 2H2O |
From this equation, we can see that the mole ratio between hydrogen gas and water is 2:2, or 1:1. This means that for every 2 moles of hydrogen gas used, 2 moles of water are produced. Similarly, the mole ratio between oxygen gas and water is 1:2. This means that for every mole of oxygen gas used, 2 moles of water are produced.
By using mole ratios, we can calculate the quantity of a substance needed or produced in a reaction, given the quantity of another substance. This allows us to understand the stoichiometry of the reaction and make predictions about the amounts of reactants and products.
Using Stoichiometry to Solve Problems

Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It is a crucial tool for chemists to determine the amount of substances involved in a reaction and to predict the outcome of a reaction. By using stoichiometry, chemists can calculate the amount of reactants needed, the amount of products that will be obtained, and even the limiting reagent in a reaction.
One of the key concepts in stoichiometry is the mole ratio. The mole ratio is the ratio of the coefficients in a balanced chemical equation. It allows chemists to convert between the quantities of different substances in a reaction. By knowing the mole ratio, chemists can calculate the mass, volume, or number of moles of a substance involved in a reaction.
When solving stoichiometry problems, it is important to follow a systematic approach. First, write a balanced chemical equation for the reaction. Then, identify the known and unknown quantities. Use the known quantities to calculate the unknown quantity using the mole ratio and the appropriate conversion factors. Finally, check the answer for correctness and significant figures.
Stoichiometry can be applied to a wide range of problems, including determining the amount of product that can be obtained from a given amount of reactant, finding the percent yield of a reaction, and calculating the concentration of a solution. It is a powerful tool that allows chemists to understand and analyze chemical reactions in a quantitative manner.
In conclusion, stoichiometry is a fundamental concept in chemistry that enables chemists to solve problems related to the amounts of reactants and products in a chemical reaction. By using the mole ratio and following a systematic approach, chemists can calculate the desired quantities and make predictions about the outcome of a reaction. Stoichiometry is an essential tool for understanding and studying chemical reactions.
Common Mistakes and Tips for Success
When it comes to studying stoichiometry and completing POGIL packets, there are some common mistakes that students tend to make. By being aware of these mistakes and following some tips for success, you can improve your understanding of the topic and achieve better results on your assignments and exams.
Common Mistakes:
- Not understanding the concept: One of the biggest mistakes students make is simply memorizing formulas and steps without understanding the underlying concept of stoichiometry. It’s important to grasp the fundamental principles to apply them correctly.
- Skipping the conversion factors: Another mistake is neglecting to include the appropriate conversion factors when solving stoichiometry problems. This can lead to incorrect calculations and ultimately incorrect answers.
- Forgetting to balance equations: Balancing chemical equations is a crucial step in stoichiometry calculations. Students often forget this step, which can throw off the entire problem-solving process.
- Not checking units: Stoichiometry involves working with different units, such as grams, moles, and liters. Forgetting to convert between units or not checking for consistency in units can lead to errors in calculations.
- Ignoring significant figures: Precision is important in stoichiometry. Ignoring or incorrectly applying significant figures can result in rounded or inaccurate answers.
Tips for Success:

- Understand the basics: Take the time to fully comprehend the basic concepts of stoichiometry. This will provide a solid foundation for solving more complex problems.
- Practice, practice, practice: Stoichiometry requires practice to become proficient. Work through as many problems as you can, using a variety of scenarios, to gain confidence and improve your problem-solving skills.
- Double-check your work: Always review your calculations and answers to ensure accuracy. Check units, conversion factors, balancing equations, and significant figures to avoid simple mistakes.
- Seek help when needed: If you’re struggling with stoichiometry or specific aspects of it, don’t hesitate to seek help. Talk to your teacher, join a study group, or seek online resources for additional support.
- Use logical reasoning: Stoichiometry problems often require logical reasoning and critical thinking. Take your time to analyze the problem, develop a plan, and think through each step before jumping into calculations.
By avoiding common mistakes and following these tips, you can improve your understanding and performance in stoichiometry. With practice and dedication, you’ll become more comfortable with the topic and be able to tackle more challenging problems with ease.