
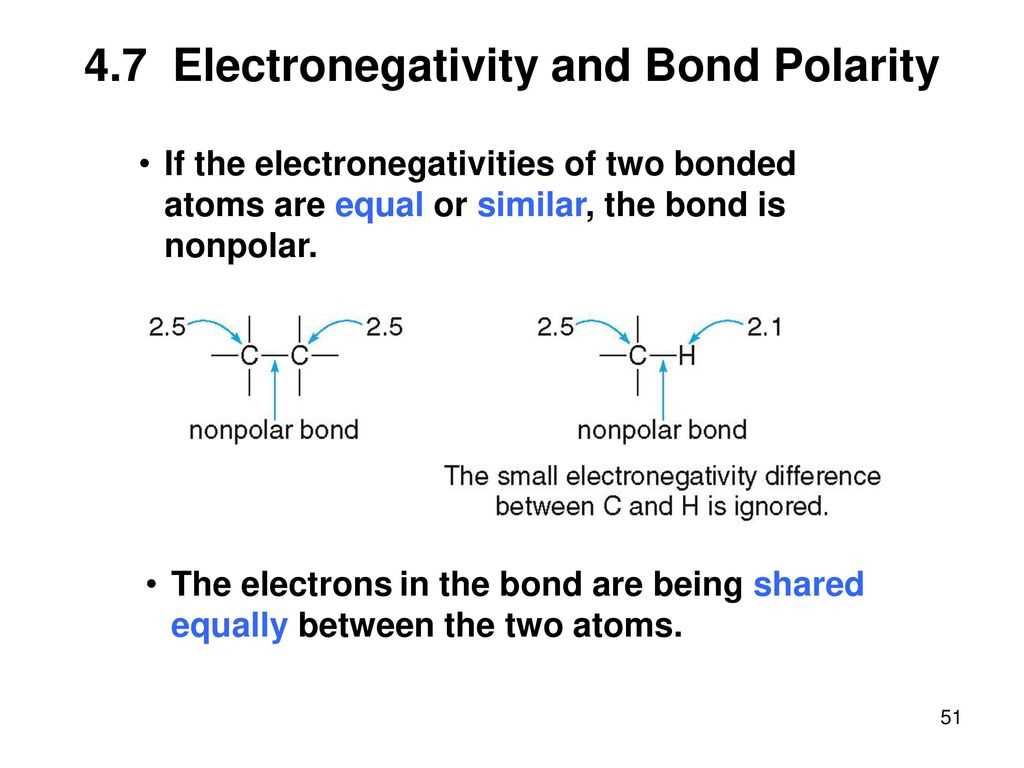
Understanding polarity and electronegativity is essential in the study of chemistry. Polarity refers to the separation of electric charges within a chemical compound caused by differences in electronegativity. Electronegativity, on the other hand, is a measure of an atom’s ability to attract electrons in a covalent bond.
When atoms share electrons unequally in a chemical bond, a polar covalent bond is formed. This means that one atom has a slightly positive charge, while the other has a slightly negative charge. The degree of polarity in a bond depends on the difference in electronegativity between the two atoms involved.
A common way to represent polarity is by using the concept of partial charges. The atom with the higher electronegativity will have a partial negative charge, symbolized by δ-, while the atom with the lower electronegativity will have a partial positive charge, symbolized by δ+. These partial charges indicate the unequal sharing of electrons in a bond.
Polarity and Electronegativity Worksheet Answers
In the world of chemistry, polarity and electronegativity are two important concepts that help us understand how atoms interact with each other. The concept of polarity refers to the separation of charge within a molecule, while electronegativity measures an atom’s ability to attract electrons towards itself in a chemical bond.
When answering a polarity and electronegativity worksheet, it is essential to understand the relationship between these two concepts. Polarity is determined by the difference in electronegativity between atoms in a molecule. If the electronegativity difference is large, the molecule is said to be polar, while if the difference is small or nonexistent, the molecule is nonpolar.
The worksheet may ask for the polarity of specific molecules, such as H2O or CO2. To determine their polarity, we need to look at the electronegativity values of the atoms within the molecule. Oxygen (O) has a higher electronegativity than hydrogen (H), so the O-H bond in water (H2O) is polar. This creates a slight negative charge on the oxygen atom and a slight positive charge on the hydrogen atoms. In the case of carbon dioxide (CO2), carbon (C) and oxygen (O) have similar electronegativities, resulting in a nonpolar molecule.
The worksheet may also include questions about the types of intermolecular forces present in certain compounds. Polarity plays a role in determining these forces. Polar molecules can form dipole-dipole interactions, where the positive end of one molecule is attracted to the negative end of another. Nonpolar molecules typically only experience London dispersion forces, which are weaker forces of attraction between temporarily induced dipoles.
In conclusion, answering a polarity and electronegativity worksheet requires an understanding of the relationship between these concepts. Polarity is determined by the difference in electronegativity, and it affects the overall charge distribution within a molecule. By knowing the electronegativity values of different atoms, we can determine the polarity of molecules and predict the types of intermolecular forces they experience.
The Concept of Polarity
The concept of polarity is a fundamental concept in chemistry. It refers to the distribution of charge within a molecule or compound. In other words, it describes how the electrons are shared between atoms within a molecule. Understanding polarity is crucial for understanding the behavior of molecules and their interactions with other substances.
When atoms bond together to form a molecule, they share electrons. However, not all atoms have an equal ability to attract electrons. Some atoms have a higher electronegativity, which means they have a stronger pull on the shared electrons. This results in an uneven distribution of charge within the molecule, creating regions of partial positive and partial negative charge. These regions are referred to as poles, and the molecule is said to be polar.
The polarity of a molecule is determined by two main factors: the electronegativity difference between the atoms and the arrangement of the atoms within the molecule. If the electronegativity difference is significant and the atoms are arranged in a way that creates an asymmetrical distribution of charge, the molecule will be polar. On the other hand, if the electronegativity difference is small or the atoms are arranged symmetrically, the molecule will be nonpolar.
Polarity plays a crucial role in many chemical processes. For example, polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents. Polarity also affects the physical properties of substances, such as boiling and melting points. Additionally, the polarity of a molecule can determine its reactivity and its ability to form intermolecular forces, such as hydrogen bonding.
Overall, the concept of polarity is essential for understanding the behavior and properties of molecules. It helps explain why certain substances interact in specific ways and provides insights into chemical reactions and molecular structures.
Understanding Electronegativity Values
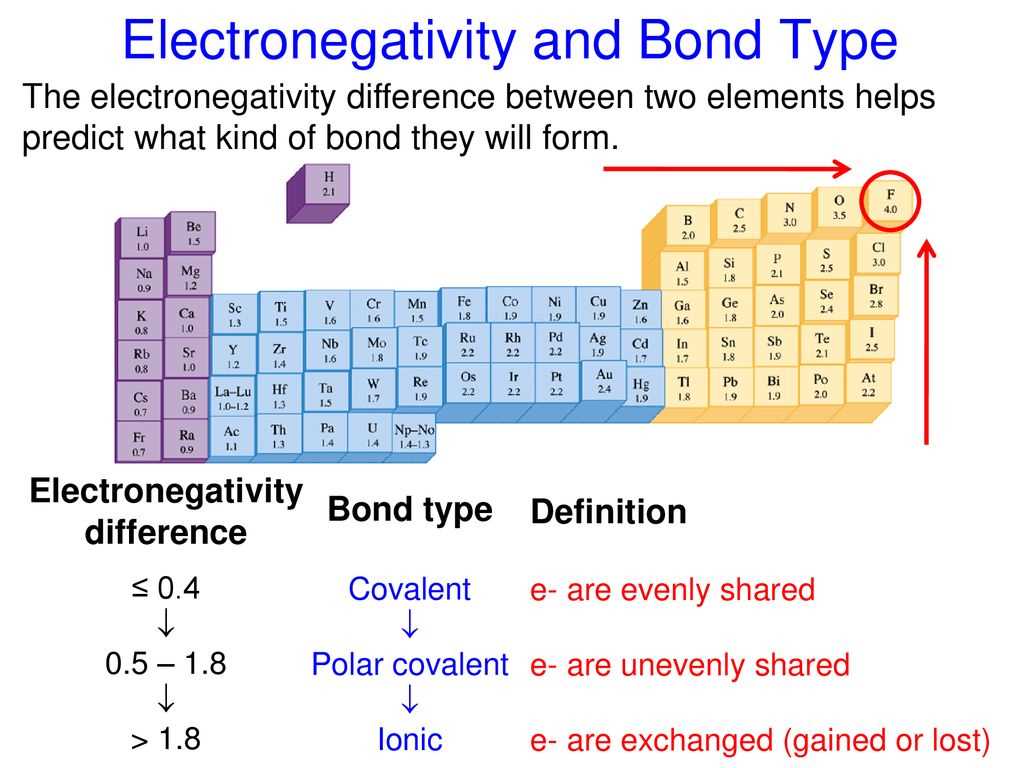
In chemistry, electronegativity is a measure of the tendency of an atom to attract electrons towards itself in a chemical bond. Electronegativity values are assigned to each element on the periodic table, which help us understand the polarity of chemical bonds and the nature of the atoms involved in the bond.
Electronegativity values range from 0 to 4, with higher values indicating a stronger ability to attract electrons. The most electronegative element is fluorine, with a value of 4, while the least electronegative element is francium, with a value close to 0. The values are determined through various experimental and theoretical methods, such as the Pauling scale and Mulliken scale.
Understanding electronegativity values is crucial in predicting the type of chemical bond that will form between two atoms. When the electronegativity difference between two atoms is large, such as in the case of a metal and a nonmetal, an ionic bond is formed. The nonmetal atom will attract the electrons more strongly, resulting in the formation of positively and negatively charged ions.
On the other hand, when the electronegativity difference is small, such as in the case of two nonmetals, a covalent bond is formed. In this type of bond, the electrons are shared between the atoms, with a partial negative charge on the atom with higher electronegativity and a partial positive charge on the other atom.
Overall, electronegativity values provide a quantitative measure of the polarity of chemical bonds and help us understand the behavior of atoms in compounds. By considering electronegativity values, scientists can make predictions about the type of bond that will form and the overall polarity of a molecule.
Polar and Nonpolar Molecules
In chemistry, molecules are classified into two main categories: polar and nonpolar. This classification is based on the distribution of electrons within a molecule and the resulting charge distribution.
Polar molecules are characterized by an unequal sharing of electrons between atoms. This occurs when there is a difference in electronegativity, or the ability of an atom to attract electrons, between the atoms in the molecule. The atom with higher electronegativity attracts the shared electrons closer to itself, creating a partial negative charge, while the other atom(s) have a partial positive charge. This uneven charge distribution results in a molecule with a positive and negative end, or poles. For example, water (H₂O) is a polar molecule because the oxygen atom is more electronegative than the hydrogen atoms, causing the oxygen end of the molecule to be slightly negative and the hydrogen end to be slightly positive.
Nonpolar molecules, on the other hand, are characterized by an equal sharing of electrons between atoms. This occurs when the atoms in a molecule have similar electronegativities, resulting in a symmetrical electron distribution and no significant charge separation. Nonpolar molecules do not have distinct positive and negative poles. An example of a nonpolar molecule is carbon dioxide (CO₂), where the carbon and oxygen atoms have similar electronegativities and the molecule has a linear shape.
In summary, the polarity of a molecule is determined by the electronegativity difference between its atoms. Polar molecules have an uneven charge distribution and distinct positive and negative poles, while nonpolar molecules have a symmetrical electron distribution and no significant charge separation.
Molecular Polarity and Dipole Moments
In chemistry, molecular polarity refers to the uneven distribution of electron density within a molecule. This distribution can result in a molecule having a positive and a negative end, known as a dipole. The polarity of a molecule is determined by the electronegativity difference between its atoms.
Electronegativity is a measure of an atom’s ability to attract electrons towards itself in a chemical bond. It is determined by various factors, such as the atomic number, distance from the nucleus, and shielding effect. When atoms with different electronegativities form a covalent bond, the electrons are shared unequally, resulting in a polar bond.
A molecule is considered polar if it has a net dipole moment, which is the product of the bond length and the difference in electronegativity between the atoms. The dipole moment is a vector quantity, meaning it has both magnitude and direction. The direction of the dipole moment is from the positive end towards the negative end.
To determine the molecular polarity, we can use the concept of the individual bond dipole moments and their arrangement within the molecule. If the individual bond dipole moments cancel out each other due to symmetrical arrangement, the molecule is nonpolar. However, if the bond dipole moments do not cancel out, the molecule is polar.
Knowing the molecular polarity is essential as it affects various properties of a substance, such as solubility, boiling point, and intermolecular forces. Understanding the concept of dipole moments helps in predicting the physical and chemical behavior of molecules and their interactions with other substances.
Predicting Molecular Polarity
In chemistry, molecular polarity refers to the distribution of charged particles or dipoles within a molecule. It is determined by the electronegativity difference between the atoms in the molecule and the molecular geometry. By understanding the concept of molecular polarity, we can predict the physical properties and chemical behavior of molecules.
One way to predict molecular polarity is by examining the individual bond polarities and the molecular geometry. A bond is polar when there is a significant difference in electronegativity between the atoms involved. This creates a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom. If the molecular geometry of the molecule is symmetrical, the bond polarities may cancel out, resulting in a nonpolar molecule. However, if the molecular geometry is asymmetrical, the bond polarities do not cancel out, leading to a polar molecule.
For example, in a molecule like carbon dioxide (CO2), the bonds between carbon and oxygen are polar due to the electronegativity difference. However, the molecule is linear, with the oxygen atoms located on opposite sides of the carbon atom. This symmetrical arrangement cancels out the individual bond polarities, resulting in a nonpolar molecule.
- Key phrase: Molecular polarity refers to the distribution of charged particles or dipoles within a molecule.
- Key phrase: Bond polarities may cancel out in a symmetrical molecular geometry, leading to a nonpolar molecule.
On the other hand, in a molecule like water (H2O), the bonds between hydrogen and oxygen are polar. The molecule has a bent molecular geometry, with the oxygen atom in the center and the hydrogen atoms on either side. This asymmetrical arrangement does not cancel out the bond polarities, resulting in a polar molecule. The oxygen atom has a partial negative charge, while the hydrogen atoms have partial positive charges.
Understanding molecular polarity is crucial in fields such as biochemistry and pharmaceuticals, as it affects the interactions between molecules and their biological activities. Additionally, knowing the polarity of a molecule helps determine its solubility in different solvents and its behavior in chemical reactions.
In conclusion, predicting molecular polarity involves analyzing the electronegativity differences between atoms and the molecular geometry. By considering these factors, we can determine whether a molecule is polar or nonpolar, which has significant implications for its physical and chemical properties.
Worksheet Answers and Explanations
Below are the answers and explanations for the questions on the polarity and electronegativity worksheet.
Question 1:
What is electronegativity?
Electronegativity is the measure of an atom’s ability to attract electrons towards itself in a chemical bond.
Question 2:
Which atom is more electronegative in a bond between hydrogen and oxygen?
Oxygen is more electronegative than hydrogen. This means that oxygen attracts the shared electrons in the bond more strongly than hydrogen, creating a polar covalent bond.
Question 3:
What is the polarity of a molecule?
The polarity of a molecule is determined by the presence of polar bonds and the molecular geometry. If a molecule has polar bonds and an asymmetrical molecular shape, it will be a polar molecule. If a molecule has nonpolar bonds or a symmetrical molecular shape, it will be a nonpolar molecule.
Question 4:
Is carbon dioxide (CO2) a polar or nonpolar molecule?
Carbon dioxide is a nonpolar molecule because it has a linear molecular geometry and the bond dipoles cancel each other out due to their symmetry.
Question 5:
Is ammonia (NH3) a polar or nonpolar molecule?
Ammonia is a polar molecule because it has a trigonal pyramidal molecular geometry and the bond dipoles do not cancel each other out.
Question 6:
What is the difference between nonpolar covalent, polar covalent, and ionic bonds?
- Nonpolar covalent bonds: The electrons are equally shared between two atoms, resulting in a balanced distribution of charge.
- Polar covalent bonds: The electrons are unequally shared between two atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other.
- Ionic bonds: One atom completely transfers electrons to another, resulting in a complete transfer of charge and the formation of ions.
Overall, understanding the concepts of polarity and electronegativity is crucial in predicting the behavior of molecules and their interactions. By analyzing the electronegativity difference between atoms and the molecular geometry, one can determine the polarity of a molecule and predict its reactivity and solubility.